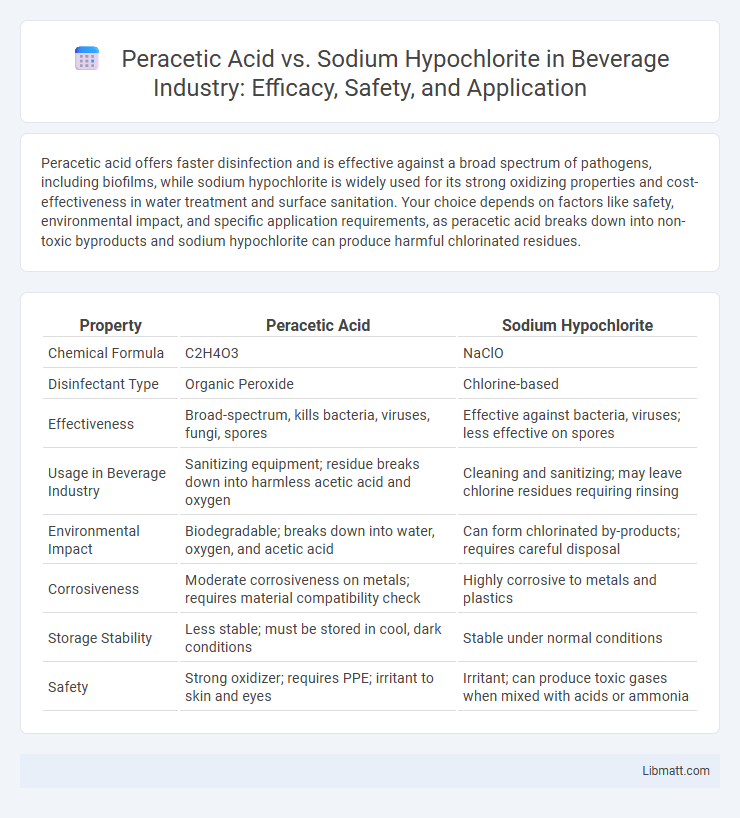
Peracetic acid offers faster disinfection and is effective against a broad spectrum of pathogens, including biofilms, while sodium hypochlorite is widely used for its strong oxidizing properties and cost-effectiveness in water treatment and surface sanitation. Your choice depends on factors like safety, environmental impact, and specific application requirements, as peracetic acid breaks down into non-toxic byproducts and sodium hypochlorite can produce harmful chlorinated residues.
Table of Comparison
| Property | Peracetic Acid | Sodium Hypochlorite |
|---|---|---|
| Chemical Formula | C2H4O3 | NaClO |
| Disinfectant Type | Organic Peroxide | Chlorine-based |
| Effectiveness | Broad-spectrum, kills bacteria, viruses, fungi, spores | Effective against bacteria, viruses; less effective on spores |
| Usage in Beverage Industry | Sanitizing equipment; residue breaks down into harmless acetic acid and oxygen | Cleaning and sanitizing; may leave chlorine residues requiring rinsing |
| Environmental Impact | Biodegradable; breaks down into water, oxygen, and acetic acid | Can form chlorinated by-products; requires careful disposal |
| Corrosiveness | Moderate corrosiveness on metals; requires material compatibility check | Highly corrosive to metals and plastics |
| Storage Stability | Less stable; must be stored in cool, dark conditions | Stable under normal conditions |
| Safety | Strong oxidizer; requires PPE; irritant to skin and eyes | Irritant; can produce toxic gases when mixed with acids or ammonia |
Introduction to Peracetic Acid and Sodium Hypochlorite
Peracetic acid is a potent oxidizing agent widely used as a disinfectant and sanitizer in food processing and healthcare due to its rapid antimicrobial activity and eco-friendly degradation into non-toxic byproducts. Sodium hypochlorite, commonly known as bleach, serves as a strong chlorine-based disinfectant with broad-spectrum efficacy but poses challenges related to stability, corrosiveness, and harmful chlorinated byproducts. Both chemicals are integral to sanitation protocols, with peracetic acid favored for residue-free applications and sodium hypochlorite valued for cost-effectiveness and availability.
Chemical Properties and Composition
Peracetic acid is a powerful oxidizing agent composed of acetic acid and hydrogen peroxide, known for its broad-spectrum antimicrobial properties and rapid decomposition into environmentally safe byproducts. Sodium hypochlorite contains chlorine in a hypochlorite ion form, providing strong disinfecting capabilities through the release of free chlorine, but it can produce harmful chlorinated compounds during degradation. Understanding the chemical composition of these agents helps you select the appropriate disinfectant based on stability, efficacy, and environmental impact.
Mechanism of Action: How Each Disinfectant Works
Peracetic acid acts by releasing free radicals that disrupt cell membranes, proteins, and essential enzymes, leading to rapid microbial cell death. Sodium hypochlorite kills microorganisms through oxidation by hypochlorous acid, which penetrates cells and denatures proteins and enzymes. Your choice between these disinfectants depends on the specific microbial load and surface compatibility, given their distinct mechanisms of action.
Common Applications in Industry and Healthcare
Peracetic acid and sodium hypochlorite serve crucial roles in industry and healthcare, with peracetic acid favored for sterilizing medical instruments and disinfecting food processing equipment due to its strong oxidizing properties and biodegradability. Sodium hypochlorite is widely used for surface disinfection, water treatment, and sanitation in hospitals and industrial facilities because of its effectiveness against a broad spectrum of pathogens and cost efficiency. Your choice between these disinfectants depends on factors like application environment, target microorganisms, and safety concerns.
Efficacy Against Bacteria, Viruses, and Fungi
Peracetic acid demonstrates superior efficacy against a broad spectrum of bacteria, viruses, and fungi due to its strong oxidizing properties, rapidly disrupting cellular membranes and viral envelopes. Sodium hypochlorite effectively inactivates many pathogens but can be less stable in the presence of organic matter and has variable activity against fungal spores. Both agents are widely used in disinfection, but peracetic acid offers faster action and a wider antimicrobial range, making it highly effective for critical sterilization applications.
Safety and Environmental Impact Comparison
Peracetic acid offers a safer profile compared to sodium hypochlorite, as it breaks down into non-toxic byproducts like water, oxygen, and acetic acid, reducing harmful environmental residues. Sodium hypochlorite poses greater risks due to its potential to release toxic chlorine gas and form carcinogenic chlorinated compounds during degradation. Your choice of disinfectant impacts both operator safety and environmental sustainability, with peracetic acid presenting a more eco-friendly and less hazardous option.
Handling, Storage, and Stability
Peracetic acid requires careful handling due to its strong oxidizing properties and potential for skin and respiratory irritation, necessitating storage in a cool, well-ventilated area away from heat and organic materials to maintain stability. Sodium hypochlorite solutions are easier to handle but degrade more rapidly when exposed to light, heat, and air, requiring opaque containers and stable temperatures for effective storage. You should choose peracetic acid for applications demanding high disinfectant stability under varied conditions, while sodium hypochlorite offers simpler handling but demands frequent replenishment to preserve efficacy.
Cost-Effectiveness and Availability
Peracetic acid offers higher disinfection efficacy at lower concentrations, which can reduce overall usage and long-term operational costs compared to sodium hypochlorite. Sodium hypochlorite is widely available and typically less expensive upfront, making it a popular choice for large-scale water treatment and sanitation. The cost-effectiveness of peracetic acid depends on factors like application frequency and storage requirements, while sodium hypochlorite benefits from established supply chains and easier handling.
Regulatory Guidelines and Compliance
Peracetic acid and sodium hypochlorite each have distinct regulatory guidelines due to their chemical properties and environmental impact. Peracetic acid is often favored in industries requiring stringent disinfection standards with fewer harmful byproducts, aligning with EPA and FDA regulations for food processing and healthcare settings. Your choice should consider compliance with OSHA standards and local environmental agencies to ensure safe usage, storage, and disposal practices for either disinfectant.
Choosing the Right Disinfectant: Key Considerations
Peracetic acid offers rapid, broad-spectrum antimicrobial activity effective against bacteria, viruses, and spores, making it ideal for high-level disinfection in healthcare and food processing. Sodium hypochlorite is a cost-effective and widely used disinfectant with strong oxidizing properties but can produce harmful byproducts and corrode surfaces over time. Your choice depends on factors like application environment, contact time, surface compatibility, and safety requirements to ensure effective pathogen control without damaging equipment.
Peracetic acid vs sodium hypochlorite Infographic
 libmatt.com
libmatt.com