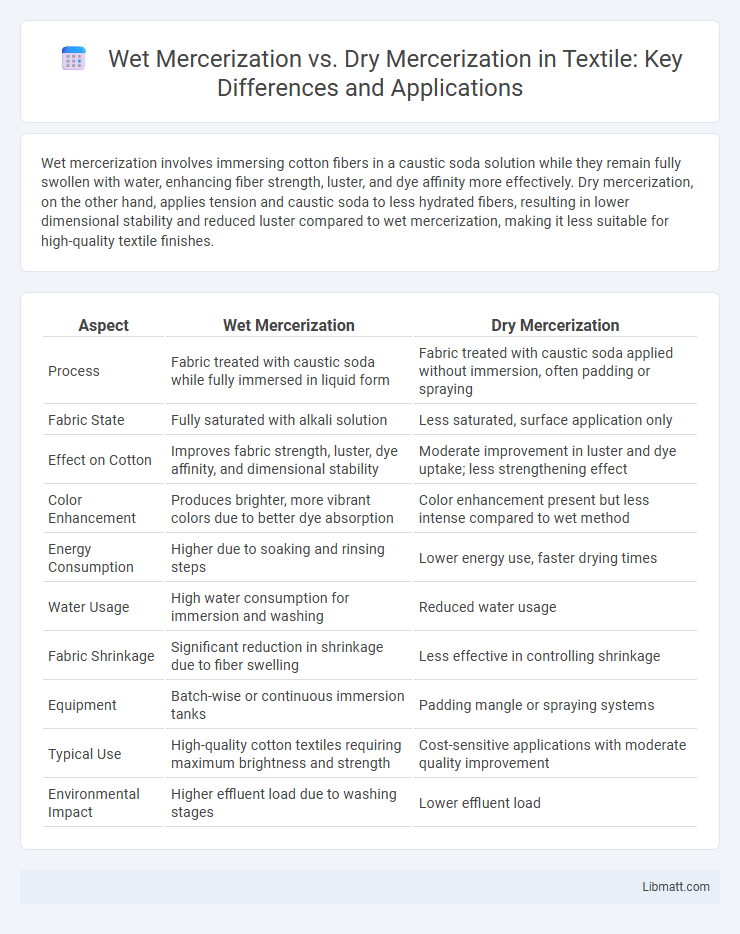
Wet mercerization involves immersing cotton fibers in a caustic soda solution while they remain fully swollen with water, enhancing fiber strength, luster, and dye affinity more effectively. Dry mercerization, on the other hand, applies tension and caustic soda to less hydrated fibers, resulting in lower dimensional stability and reduced luster compared to wet mercerization, making it less suitable for high-quality textile finishes.
Table of Comparison
| Aspect | Wet Mercerization | Dry Mercerization |
|---|---|---|
| Process | Fabric treated with caustic soda while fully immersed in liquid form | Fabric treated with caustic soda applied without immersion, often padding or spraying |
| Fabric State | Fully saturated with alkali solution | Less saturated, surface application only |
| Effect on Cotton | Improves fabric strength, luster, dye affinity, and dimensional stability | Moderate improvement in luster and dye uptake; less strengthening effect |
| Color Enhancement | Produces brighter, more vibrant colors due to better dye absorption | Color enhancement present but less intense compared to wet method |
| Energy Consumption | Higher due to soaking and rinsing steps | Lower energy use, faster drying times |
| Water Usage | High water consumption for immersion and washing | Reduced water usage |
| Fabric Shrinkage | Significant reduction in shrinkage due to fiber swelling | Less effective in controlling shrinkage |
| Equipment | Batch-wise or continuous immersion tanks | Padding mangle or spraying systems |
| Typical Use | High-quality cotton textiles requiring maximum brightness and strength | Cost-sensitive applications with moderate quality improvement |
| Environmental Impact | Higher effluent load due to washing stages | Lower effluent load |
Introduction to Mercerization
Mercerization is a textile finishing process that enhances cotton fiber properties by treating it with a strong caustic soda solution. Wet mercerization involves immersing the cotton fibers in the caustic solution while retaining water, leading to increased fiber swelling, luster, and dye affinity. Dry mercerization uses a controlled drying phase during treatment, resulting in improved fabric dimensional stability and a softer hand feel compared to wet mercerization.
Overview of Wet and Dry Mercerization
Wet mercerization involves treating cotton fibers with a strong alkali solution while they are fully saturated with water, enhancing fiber strength, luster, and dye affinity. Dry mercerization, on the other hand, applies alkali to fibers with minimal moisture content, resulting in a less uniform effect but faster processing time. Your choice between wet and dry mercerization impacts the fabric's appearance, durability, and dye uptake based on the specific textile application.
Chemical Process Involved in Each Method
Wet mercerization involves treating cotton fibers with a concentrated sodium hydroxide (NaOH) solution while the fibers are under tension, causing fiber swelling, increased crystallinity, and enhanced dye affinity through the reversible transformation of cellulose I to cellulose II. Dry mercerization, on the other hand, uses a similar NaOH treatment but with reduced moisture content and without tension, leading to less uniform chemical penetration and cellulose modification. The chemical interaction in both methods centers on the alkali-induced disruption of hydrogen bonds in cellulose, but the water presence and mechanical tension in wet mercerization optimize fiber swelling and cellulose reorganization more effectively.
Equipment and Machinery Differences
Wet mercerization utilizes immersion tanks and specialized washing machines to maintain continuous water saturation, while dry mercerization requires heated chambers or steam ovens to control temperature and humidity precisely. Equipment for wet mercerization often includes water circulation systems and chemical dosing units, contrasting with dry mercerization machinery that emphasizes controlled air flow and thermal regulation. These distinct machinery requirements influence the operational scale, energy consumption, and process efficiency in textile manufacturing.
Impacts on Fiber Structure
Wet mercerization causes fibers to swell uniformly, increasing their surface area and enhancing dye absorption while improving tensile strength and luster. Dry mercerization, in contrast, results in less swelling, maintaining more of the fiber's original rigidity but offering moderate improvements in dye affinity and strength. Understanding these structural changes helps you select the right process to optimize textile quality and performance.
Effects on Dye Affinity
Wet mercerization enhances dye affinity by swelling cellulose fibers, increasing their surface area and accessibility for dye molecules, resulting in deeper and more uniform coloration. Dry mercerization, involving less fiber swelling, produces moderate improvements in dye uptake but retains more fiber strength compared to the wet process. The increased crystallinity and reduced amorphous regions from wet mercerization significantly boost reactive dye fixation on cotton fabrics.
Strength and Durability Comparisons
Wet mercerization enhances fiber strength by swelling cotton with caustic soda, resulting in increased tensile strength and improved durability compared to untreated fibers. Dry mercerization, which applies sodium hydroxide without water, produces less fiber swelling and thus offers moderate strength gains but inferior durability relative to wet mercerization. Your choice between the two processes impacts the longevity and performance of cotton textiles, with wet mercerization being the superior method for strength and durability.
Cost and Energy Consumption
Wet mercerization generally incurs higher costs due to prolonged water usage and increased chemical treatment, resulting in greater water consumption and wastewater management expenses. Dry mercerization reduces water use significantly, lowering overall energy consumption and operational costs by relying on controlled heat and tension without extensive soaking. Your choice between these methods should consider long-term savings on energy bills and environmental impact alongside initial process costs.
Industrial Applications and Preferences
Wet mercerization is predominantly favored in textile industries for cotton fabric finishing due to its ability to enhance fiber strength, dye affinity, and luster, making it ideal for high-quality apparel production. Dry mercerization, while less common, is preferred in settings requiring faster processing and reduced chemical use, often applied in specialized technical textiles and nonwoven materials where dimensional stability is crucial. Industrial preferences lean towards wet mercerization for large-scale fabric treatment, whereas dry mercerization suits niche applications demanding eco-friendly, energy-efficient processes.
Conclusion: Selecting the Right Mercerization Method
Wet mercerization enhances fabric strength, luster, and dye affinity by treating fibers with aqueous sodium hydroxide under tension, making it ideal for high-quality cotton textiles. Dry mercerization, involving moisture-free alkali application, offers faster processing but may result in less uniform fiber modification and lower fabric quality. Your choice depends on balancing production speed with desired fabric properties, prioritizing wet mercerization for superior durability and appearance in premium products.
Wet mercerization vs Dry mercerization Infographic
 libmatt.com
libmatt.com