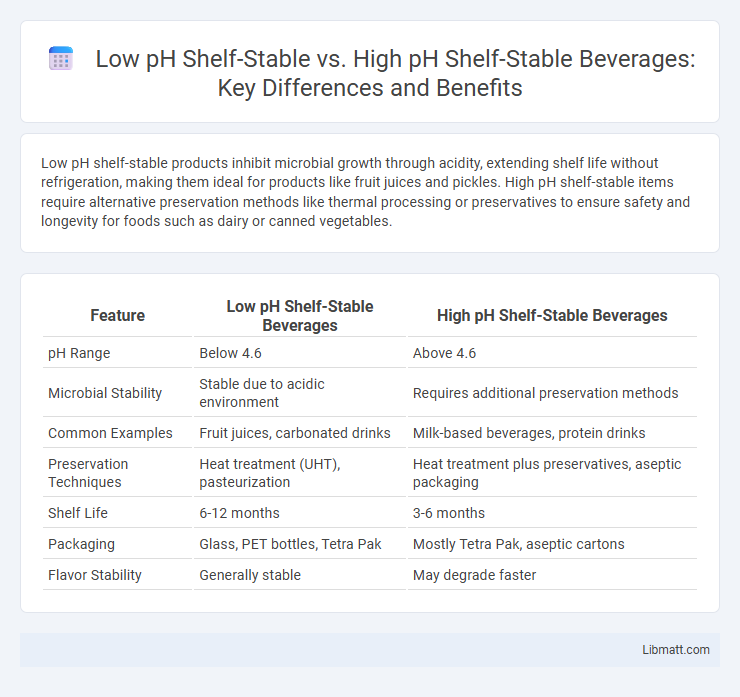
Low pH shelf-stable products inhibit microbial growth through acidity, extending shelf life without refrigeration, making them ideal for products like fruit juices and pickles. High pH shelf-stable items require alternative preservation methods like thermal processing or preservatives to ensure safety and longevity for foods such as dairy or canned vegetables.
Table of Comparison
| Feature | Low pH Shelf-Stable Beverages | High pH Shelf-Stable Beverages |
|---|---|---|
| pH Range | Below 4.6 | Above 4.6 |
| Microbial Stability | Stable due to acidic environment | Requires additional preservation methods |
| Common Examples | Fruit juices, carbonated drinks | Milk-based beverages, protein drinks |
| Preservation Techniques | Heat treatment (UHT), pasteurization | Heat treatment plus preservatives, aseptic packaging |
| Shelf Life | 6-12 months | 3-6 months |
| Packaging | Glass, PET bottles, Tetra Pak | Mostly Tetra Pak, aseptic cartons |
| Flavor Stability | Generally stable | May degrade faster |
Introduction to Shelf-Stable Foods
Shelf-stable foods with low pH, typically below 4.6, inhibit microbial growth through acidity, ensuring safety without refrigeration and extending shelf life. High pH shelf-stable products rely on other preservation methods such as thermal processing or additives to prevent spoilage, as their neutral or alkaline pH allows for faster microbial growth. Understanding the difference between low pH and high pH shelf-stable foods helps you select appropriate storage and usage practices to maintain food quality and safety.
Understanding pH: Low vs High pH Foods
Low pH shelf-stable foods, typically below 4.6, inhibit bacterial growth by creating an acidic environment that preserves freshness and extends shelf life. High pH shelf-stable foods, with pH values above 4.6, require additional processing methods such as pressure canning to prevent spoilage from harmful microorganisms. Understanding the acidity level of your food is crucial for selecting the appropriate preservation technique to ensure safety and maintain quality.
The Science of Food Preservation and pH
Low pH shelf-stable foods, typically below pH 4.6, inhibit the growth of harmful bacteria such as Clostridium botulinum by creating an acidic environment that preserves flavor and extends shelf life without refrigeration. High pH shelf-stable foods, with pH values above 4.6, require more rigorous preservation methods like thermal processing to ensure microbial safety due to their less acidic, more favorable environment for bacterial growth. Understanding the pH level is critical in food preservation science, as it determines the appropriate sterilization techniques and packaging needed to maintain food safety and quality.
Common Examples: Low pH Shelf-Stable Products
Low pH shelf-stable products include fruit juices, canned tomatoes, pickles, and vinegar-based condiments, which have a pH below 4.6 that inhibits the growth of harmful bacteria such as Clostridium botulinum. These acidic environments enable preservation without refrigeration, enhancing shelf stability and safety. Low pH products rely on acidification as a primary preservation method, differing from high pH shelf-stable foods that often require additional treatments like thermal processing.
Common Examples: High pH Shelf-Stable Products
High pH shelf-stable products often include canned vegetables, such as green beans and corn, as well as canned meats and seafood like chicken and tuna, which rely on thermal processing to inhibit microbial growth. These products maintain safety and quality at pH levels above 4.6, where heat-resistant spores and bacteria pose significant risks. Understanding pH levels aids in selecting appropriate preservation methods to ensure long shelf life and food safety.
Processing Methods for Low pH Foods
Processing methods for low pH shelf-stable foods focus on acidification and thermal treatments to inhibit microbial growth and ensure safety. Techniques such as pasteurization and hot-filling are commonly used to maintain product stability while preserving flavor and nutrients. Your understanding of these methods helps in selecting appropriate processing strategies for acidic foods like fruit juices and pickles.
Processing Methods for High pH Foods
High pH shelf-stable foods require more intensive thermal processing techniques such as retorting and pressure cooking to inhibit microbial growth and ensure safety. These foods typically undergo higher temperature and longer time treatments compared to low pH products to inactivate heat-resistant pathogens like Clostridium botulinum. Advanced sterilization methods, including aseptic processing and ultra-high temperature (UHT) treatment, are also employed to maintain quality while achieving effective microbial control in high pH shelf-stable foods.
Safety Considerations: Microbial Risks and pH
Low pH shelf-stable foods typically inhibit microbial growth due to their acidic environment, significantly reducing safety concerns related to pathogens like Clostridium botulinum. High pH shelf-stable foods require stringent preservation methods, such as thermal processing, to prevent microbial risks associated with bacteria and spoilage organisms thriving in less acidic conditions. Your food safety protocols should prioritize pH measurement and appropriate preservation techniques to ensure product stability and minimize health hazards.
Regulatory Standards for pH in Shelf-Stable Foods
Regulatory standards for pH in shelf-stable foods categorize products into low pH (<=4.6) and high pH (>4.6) groups, which determine safety protocols and processing methods. Low pH shelf-stable foods, such as pickles and fruit juices, undergo acidification processes to inhibit microbial growth and require less stringent thermal processing compared to high pH foods like canned vegetables and meats, which demand rigorous sterilization to prevent Clostridium botulinum contamination. Understanding these pH-based regulatory requirements helps you ensure compliance and maintain food safety throughout shelf-stable product development.
Consumer Considerations: Taste, Texture, and Nutrition
Low pH shelf-stable products typically offer a tangy or acidic taste profile that can enhance flavor perception, while high pH shelf-stable items tend to have a milder, less sharp taste. Texture in low pH products often remains firmer due to acidic preservation, whereas high pH products may exhibit a softer consistency as a result of alkaline conditions affecting cell structure. Nutritionally, low pH foods can better retain certain vitamins like vitamin C and antioxidants, while high pH environments may lead to nutrient degradation but can help preserve protein integrity.
Low pH shelf-stable vs high pH shelf-stable Infographic
 libmatt.com
libmatt.com