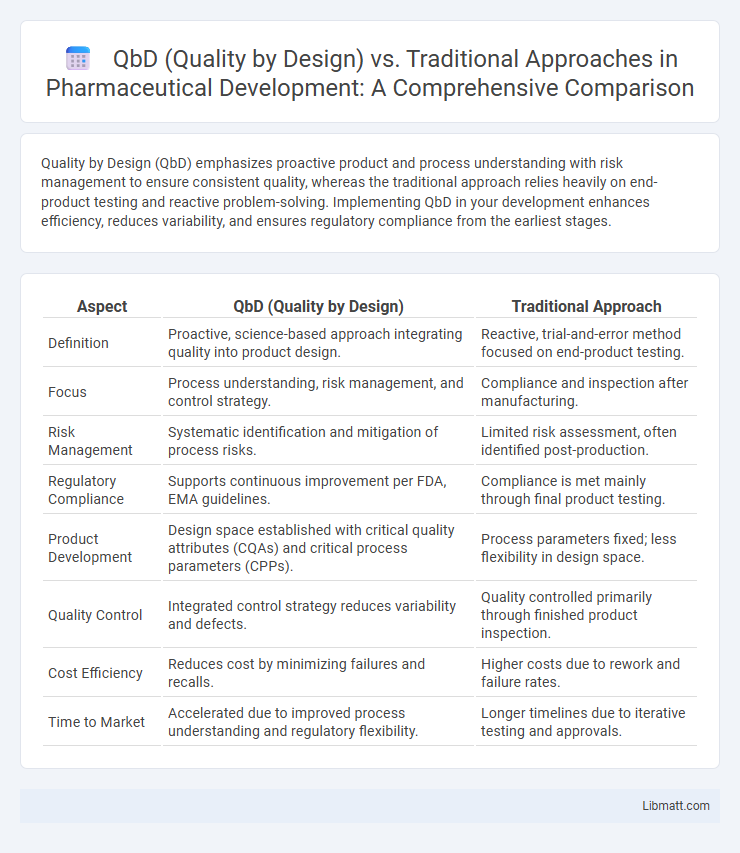
Quality by Design (QbD) emphasizes proactive product and process understanding with risk management to ensure consistent quality, whereas the traditional approach relies heavily on end-product testing and reactive problem-solving. Implementing QbD in your development enhances efficiency, reduces variability, and ensures regulatory compliance from the earliest stages.
Table of Comparison
| Aspect | QbD (Quality by Design) | Traditional Approach |
|---|---|---|
| Definition | Proactive, science-based approach integrating quality into product design. | Reactive, trial-and-error method focused on end-product testing. |
| Focus | Process understanding, risk management, and control strategy. | Compliance and inspection after manufacturing. |
| Risk Management | Systematic identification and mitigation of process risks. | Limited risk assessment, often identified post-production. |
| Regulatory Compliance | Supports continuous improvement per FDA, EMA guidelines. | Compliance is met mainly through final product testing. |
| Product Development | Design space established with critical quality attributes (CQAs) and critical process parameters (CPPs). | Process parameters fixed; less flexibility in design space. |
| Quality Control | Integrated control strategy reduces variability and defects. | Quality controlled primarily through finished product inspection. |
| Cost Efficiency | Reduces cost by minimizing failures and recalls. | Higher costs due to rework and failure rates. |
| Time to Market | Accelerated due to improved process understanding and regulatory flexibility. | Longer timelines due to iterative testing and approvals. |
Introduction to QbD and Traditional Approaches
Quality by Design (QbD) is a systematic approach to pharmaceutical development that emphasizes designing processes and controls based on scientific understanding and risk management. Traditional approaches rely heavily on end-product testing and predefined procedures without comprehensive process insight. Your pharmaceutical quality management benefits significantly from QbD by enhancing product consistency, reducing variability, and ensuring regulatory compliance from the development phase onward.
Defining Quality by Design (QbD)
Quality by Design (QbD) is a systematic approach to pharmaceutical development that emphasizes designing quality into products from the outset through predefined objectives and understanding of processes and materials. Unlike the traditional approach that relies heavily on end-product testing to ensure quality, QbD integrates risk management, scientific principles, and process control to predict and mitigate potential quality issues. This results in improved product consistency, reduced variability, and enhanced regulatory compliance by embedding quality throughout the product life cycle.
Overview of the Traditional Pharmaceutical Development Approach
The traditional pharmaceutical development approach relies heavily on trial-and-error experimentation to define product quality, often leading to longer timelines and increased costs. Fixed formulations and processes are established based on end-product testing rather than understanding critical quality attributes and process parameters. This method lacks the systematic risk management and process design integration found in Quality by Design (QbD), resulting in less predictable product performance and regulatory challenges.
Key Principles of QbD
Quality by Design (QbD) emphasizes a systematic approach to pharmaceutical development by integrating predefined objectives, risk assessment, and robust process controls to ensure product quality. Key principles include designing quality into the process from the outset, understanding the impact of raw materials and manufacturing variables, and establishing a control strategy based on scientific knowledge and product performance criteria. This contrasts with the traditional approach, which relies heavily on end-product testing rather than proactive design and risk mitigation throughout development.
Major Differences Between QbD and Traditional Methods
Quality by Design (QbD) centers on a comprehensive understanding of processes through predefined objectives, risk assessment, and robust design frameworks, contrasting with the traditional approach that relies heavily on end-product testing and empirical knowledge. QbD employs systematic experimentation and statistical models to ensure consistent quality, whereas traditional methods often depend on trial-and-error and reactive adjustments. Your ability to anticipate variability and embed quality into the design phase distinguishes QbD as a proactive, science-based strategy compared to the reactive nature of traditional approaches.
Benefits of Implementing QbD
Implementing Quality by Design (QbD) enhances product consistency and reduces variability by emphasizing risk management and detailed process understanding from development through production. This approach enables more efficient regulatory compliance and faster product approval by providing robust design space and control strategy documentation. QbD ultimately lowers manufacturing costs and minimizes product recalls compared to the traditional approach, which relies heavily on end-product testing and reactive quality control.
Drawbacks and Limitations of the Traditional Approach
The traditional approach to product development often relies on trial-and-error methods, leading to inconsistent quality and extended timelines. This approach lacks a systematic understanding of critical process variables, resulting in increased risk of product failure and regulatory non-compliance. You may face higher costs and delayed market entry due to insufficient process control and limited ability to predict product performance.
Regulatory Perspectives on QbD vs Traditional Approach
Regulatory perspectives emphasize Quality by Design (QbD) as a proactive framework that enhances product understanding and process control through scientific risk management, contrasting with the traditional approach's reliance on end-product testing. Agencies like the FDA and EMA advocate QbD for its potential to improve product quality, reduce recalls, and facilitate regulatory flexibility via detailed design space justification. Traditional regulatory approaches focus on compliance with predefined specifications without extensive product or process insights, often leading to reactive quality assurance measures.
Case Studies: QbD Success Stories vs Traditional Challenges
Case studies reveal that Quality by Design (QbD) implementations consistently achieve higher process reliability and reduced product recalls compared to traditional methods, which often face challenges such as variability and delayed defect detection. Companies employing QbD report shorter time-to-market and enhanced regulatory compliance due to risk-based decision-making and robust design space definition. Your organization can leverage these success stories to improve product quality and operational efficiency beyond the limitations observed in traditional trial-and-error approaches.
Future Trends in Pharmaceutical Quality Management
Quality by Design (QbD) integrates systematic risk assessment and robust process understanding, driving innovation in pharmaceutical quality management by enabling real-time product monitoring and control. Future trends emphasize digital transformation through artificial intelligence, machine learning, and continuous manufacturing, which enhance predictive quality analytics and reduce variability compared to the traditional trial-and-error approach. Regulatory agencies increasingly support QbD frameworks, promoting lifecycle management and data-driven decision-making to ensure consistent product efficacy and safety.
QbD (Quality by Design) vs traditional approach Infographic
 libmatt.com
libmatt.com