A comparator arm in a clinical trial tests an alternative treatment or intervention against the experimental therapy, while the control arm typically receives a placebo or standard of care to serve as a baseline for comparison. Understanding the differences between comparator and control arms helps ensure Your study design accurately measures the efficacy and safety of the new treatment.
Table of Comparison
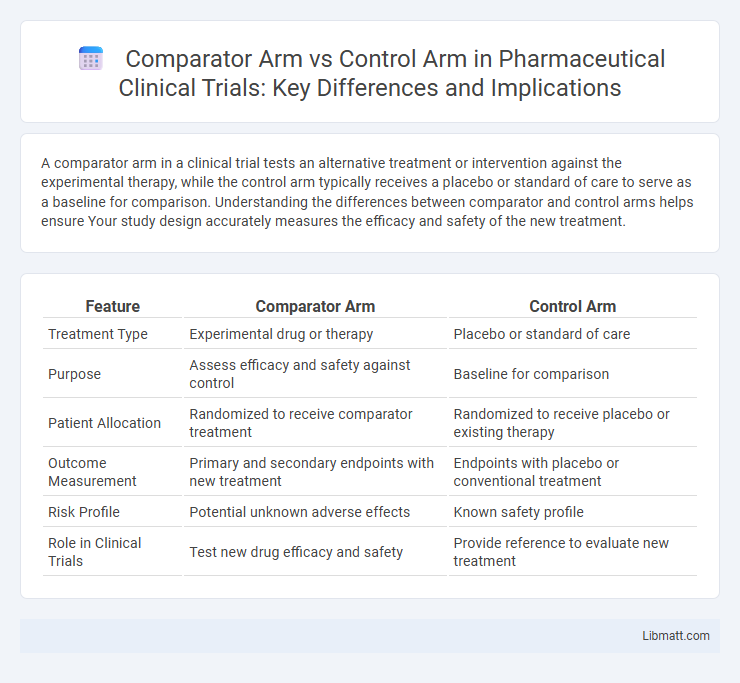
| Feature | Comparator Arm | Control Arm |
|---|---|---|
| Treatment Type | Experimental drug or therapy | Placebo or standard of care |
| Purpose | Assess efficacy and safety against control | Baseline for comparison |
| Patient Allocation | Randomized to receive comparator treatment | Randomized to receive placebo or existing therapy |
| Outcome Measurement | Primary and secondary endpoints with new treatment | Endpoints with placebo or conventional treatment |
| Risk Profile | Potential unknown adverse effects | Known safety profile |
| Role in Clinical Trials | Test new drug efficacy and safety | Provide reference to evaluate new treatment |
Introduction to Comparator and Control Arms
Comparator arms in clinical trials serve as benchmark groups receiving either standard treatment or placebo, enabling the evaluation of new interventions' effectiveness. Control arms specifically function as the baseline, providing a reference to measure the experimental treatment's impact by isolating variables. Your understanding of these arms is crucial for interpreting trial outcomes and assessing the validity of clinical research results.
Definitions: Comparator Arm vs Control Arm
The comparator arm in a clinical trial refers to the group receiving an alternative treatment or intervention against which the experimental treatment is evaluated. The control arm typically receives a placebo, standard of care, or no treatment to serve as a baseline for comparison. Distinguishing between comparator and control arms is essential for interpreting trial results and assessing the efficacy and safety of new therapies.
The Role of Comparator Arms in Clinical Trials
Comparator arms in clinical trials serve as essential benchmarks, enabling the evaluation of a new treatment's efficacy and safety against existing standards or placebos. These arms help isolate the effects of the investigational intervention by providing controlled conditions, thereby reducing bias and increasing the reliability of study outcomes. The data derived from comparator arms are crucial for regulatory approvals and informed clinical decision-making.
Purpose of Control Arms in Study Design
Control arms in study design serve as the benchmark group that receives the standard treatment or placebo, enabling researchers to measure the efficacy and safety of the comparator arm's intervention. By isolating the effects of the experimental treatment, control arms help minimize bias and confounding variables, ensuring the validity and reliability of clinical trial results. This comparison allows for statistically significant conclusions about the new treatment's benefits and risks relative to existing standards.
Key Differences Between Comparator and Control Arms
Comparator arms receive a specific treatment or intervention being tested in a clinical trial, while control arms typically receive a placebo, standard treatment, or no intervention to provide baseline data. Comparator arms are essential for directly evaluating the efficacy and safety of new therapies against existing options, whereas control arms help isolate the effect of the investigational treatment by minimizing bias. Understanding the key differences between comparator and control arms allows you to interpret clinical trial results accurately and assess the comparative benefit of new medical interventions.
Criteria for Selecting a Comparator Arm
Selecting a comparator arm involves evaluating factors such as the relevance to current standard of care, ethical considerations, and clinical trial objectives. Your choice should align with the target population's characteristics and regulatory requirements to ensure meaningful, interpretable outcomes. Understanding the differences between comparator and control arms helps optimize trial design for accurate efficacy and safety assessment.
Ethical Considerations in Using Control Arms
Ethical considerations in using control arms center on ensuring patient safety and informed consent, particularly when withholding an experimental treatment may impact outcomes. Control arms must reflect the current standard of care to avoid exposing participants to inferior treatments or unnecessary risks. Balancing scientific rigor with participant welfare requires transparent communication and ethical oversight by institutional review boards (IRBs).
Impact on Trial Outcomes: Comparator vs Control
The choice between a comparator arm and a control arm significantly influences trial outcomes by determining the reference point for assessing treatment efficacy and safety. Comparator arms often use an active treatment, enabling direct comparison to existing therapies and providing clearer insights into relative effectiveness, while control arms typically use placebo or standard care, highlighting the investigational treatment's absolute effects. Understanding these distinctions helps you interpret trial results accurately and make informed decisions about therapeutic value.
Challenges in Implementing Comparator and Control Arms
Implementing comparator and control arms in clinical trials presents significant challenges including patient recruitment, ethical considerations, and maintaining blinding. Ensuring comparable baseline characteristics between arms is critical to avoid bias, while ethical concerns arise when using placebo controls if effective treatments exist. You must carefully design protocols to address these issues and ensure valid, reliable trial outcomes.
Conclusion: Optimizing Arm Selection for Clinical Research
Choosing the appropriate comparator arm versus control arm significantly impacts the validity and reliability of clinical trial outcomes. Comparator arms compare new interventions directly against existing treatments, enhancing the relevance of results to current clinical practice, while control arms often utilize placebos or standard care to establish baseline efficacy. Your strategic selection of trial arms optimizes data interpretation, improves patient safety assessments, and accelerates regulatory approvals in clinical research.
Comparator arm vs control arm Infographic
 libmatt.com
libmatt.com