Cationic dyes carry a positive charge, making them ideal for dyeing negatively charged fibers like acrylics, while anionic dyes possess a negative charge and are best suited for fibers such as wool, silk, and nylon. Understanding the difference between cationic and anionic dyes helps you select the right dye type to achieve vibrant, long-lasting colors on specific fabric types.
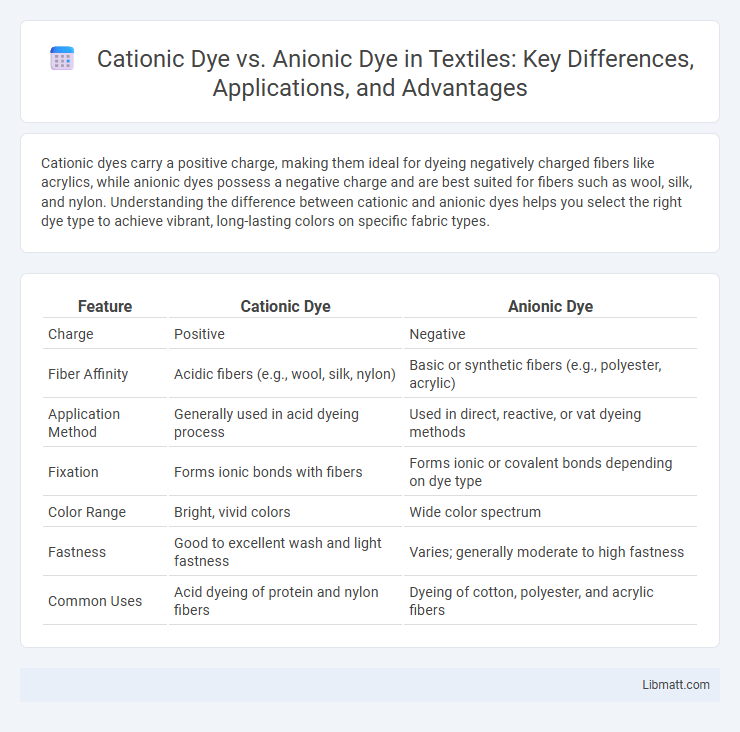
Table of Comparison
| Feature | Cationic Dye | Anionic Dye |
|---|---|---|
| Charge | Positive | Negative |
| Fiber Affinity | Acidic fibers (e.g., wool, silk, nylon) | Basic or synthetic fibers (e.g., polyester, acrylic) |
| Application Method | Generally used in acid dyeing process | Used in direct, reactive, or vat dyeing methods |
| Fixation | Forms ionic bonds with fibers | Forms ionic or covalent bonds depending on dye type |
| Color Range | Bright, vivid colors | Wide color spectrum |
| Fastness | Good to excellent wash and light fastness | Varies; generally moderate to high fastness |
| Common Uses | Acid dyeing of protein and nylon fibers | Dyeing of cotton, polyester, and acrylic fibers |
Introduction to Cationic and Anionic Dyes
Cationic dyes, also known as basic dyes, carry a positive charge and bind primarily to negatively charged substrates such as acrylic fibers and wool, making them ideal for applications in textile dyeing and biological staining. Anionic dyes possess a negative charge and are widely used for dyeing fibers like cotton and silk due to their affinity for positively charged sites in the fabric. Understanding the charge properties of these dyes is essential for selecting the appropriate dye type for specific materials and achieving optimal colorfastness and intensity.
Chemical Structure and Properties
Cationic dyes contain positively charged ions that easily bind to negatively charged substrates, making them suitable for dyeing acrylic fibers and paper. Anionic dyes, characterized by their negatively charged ions, exhibit high solubility in water and strong affinity for positively charged materials like nylon and wool. Your choice between these dyes depends on the chemical compatibility and desired fastness properties in the target application.
Key Differences Between Cationic and Anionic Dyes
Cationic dyes carry a positive charge and are primarily used for dyeing acrylic fibers, while anionic dyes possess a negative charge and are commonly applied to wool, silk, and nylon. The interaction between dye and fiber depends on charge attraction; cationic dyes bond with negatively charged fiber sites, whereas anionic dyes bond with positively charged fiber sites. Solubility and pH range also differ, with cationic dyes generally being soluble in water and stable in neutral to slightly alkaline conditions, while anionic dyes require acidic conditions for optimal fixation.
Mechanisms of Dyeing in Textiles
Cationic dyes bind to negatively charged fibers through electrostatic attraction, enhancing color fastness and brightness on materials like acrylics. Anionic dyes interact with positively charged sites on fibers such as nylon and wool, relying on ionic bonds and hydrogen bonding for durable coloration. Your choice between cationic and anionic dyes should consider fiber type and the dyeing mechanism to ensure optimal uptake and washfastness.
Common Applications of Cationic Dyes
Cationic dyes, also known as basic dyes, are commonly used in textile industries to color acrylic fibers, paper products, and leather due to their strong affinity for negatively charged substrates. These dyes are essential in producing vibrant, long-lasting colors in acrylic and polyacrylonitrile fibers, which are prevalent in clothing and upholstery. Additionally, cationic dyes find applications in biological staining and as colorants in inkjet printing and cosmetics, leveraging their vivid coloration and compatibility with various materials.
Common Uses of Anionic Dyes
Anionic dyes are extensively used in textile industries for dyeing materials such as wool, silk, and nylon due to their strong affinity for fibers carrying a positive charge. These dyes are also commonly applied in paper manufacturing and leather processing because of their excellent water solubility and vibrant color range. Their compatibility with various substrates makes anionic dyes a preferred choice for high-quality, long-lasting color applications.
Advantages and Disadvantages
Cationic dyes offer excellent affinity for synthetic fibers like acrylic, ensuring vibrant and long-lasting colors, but they often demonstrate poor fastness on natural fibers such as cotton. Anionic dyes provide superior dyeing performance on natural fibers like cotton and wool, delivering bright colors with good wash fastness, yet they may cause environmental concerns due to their sulfate or sulfonate groups. Understanding your fabric type and environmental impact helps determine whether cationic or anionic dyes suit your specific dyeing needs.
Environmental Impact and Safety
Cationic dyes, often used in textile and paper industries, can pose environmental challenges due to their persistence and toxicity to aquatic life, requiring careful wastewater treatment to prevent water pollution. Anionic dyes tend to have lower toxicity but may contribute to water pollution through high chemical oxygen demand (COD) and difficulty in biodegradation, necessitating advanced treatment methods. Your choice between cationic and anionic dyes should consider the specific environmental regulations and safety measures to minimize ecological harm.
Selection Criteria for Industrial Use
Selection criteria for industrial use of cationic dye versus anionic dye primarily depend on substrate compatibility and dyeing process requirements. Cationic dyes are preferred for acrylic fibers due to their positive charge and affinity for negatively charged fiber sites, offering vibrant colors and excellent wash fastness. Anionic dyes, such as acid and reactive dyes, are selected for materials like wool, silk, and cotton, with considerations on fiber charge, pH levels, and environmental impact guiding the choice to ensure optimal fixation and color durability.
Future Trends in Dye Chemistry
Future trends in dye chemistry emphasize the development of cationic dyes with enhanced affinity for synthetic fibers, enabling improved colorfastness and environmental sustainability. Research on anionic dyes is focusing on biodegradable and low-toxicity formulations to reduce wastewater impact from textile industries. Your choice between cationic and anionic dyes will increasingly depend on ecological considerations and fiber compatibility as green chemistry innovations advance.
Cationic Dye vs Anionic Dye Infographic
 libmatt.com
libmatt.com