Wicking corrosion occurs when moisture and contaminants are drawn into tiny gaps or cracks, causing localized metal degradation, while galvanic corrosion arises from an electrochemical reaction between two dissimilar metals in contact with an electrolyte. Understanding these differences helps you choose appropriate materials and protective measures to prevent structural damage and ensure durability.
Table of Comparison
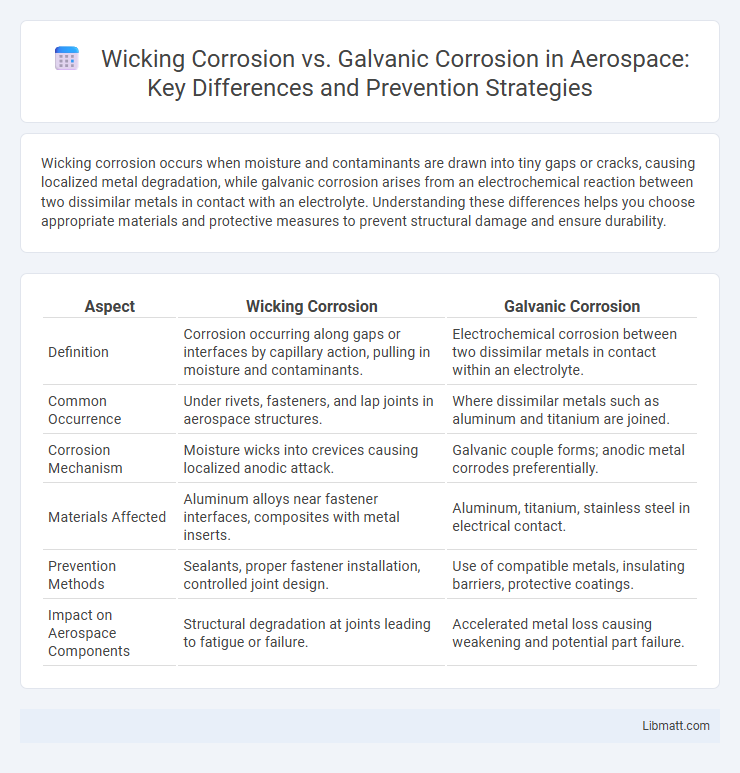
| Aspect | Wicking Corrosion | Galvanic Corrosion |
|---|---|---|
| Definition | Corrosion occurring along gaps or interfaces by capillary action, pulling in moisture and contaminants. | Electrochemical corrosion between two dissimilar metals in contact within an electrolyte. |
| Common Occurrence | Under rivets, fasteners, and lap joints in aerospace structures. | Where dissimilar metals such as aluminum and titanium are joined. |
| Corrosion Mechanism | Moisture wicks into crevices causing localized anodic attack. | Galvanic couple forms; anodic metal corrodes preferentially. |
| Materials Affected | Aluminum alloys near fastener interfaces, composites with metal inserts. | Aluminum, titanium, stainless steel in electrical contact. |
| Prevention Methods | Sealants, proper fastener installation, controlled joint design. | Use of compatible metals, insulating barriers, protective coatings. |
| Impact on Aerospace Components | Structural degradation at joints leading to fatigue or failure. | Accelerated metal loss causing weakening and potential part failure. |
Understanding Wicking Corrosion: Definition and Mechanism
Wicking corrosion occurs when electrolyte travels along a metal-to-metal or metal-to-nonmetal interface, causing localized damage beneath protective coatings or sealants. This process enables corrosion to propagate in hidden areas, often leading to structural weakness without visible surface damage. Understanding wicking corrosion's mechanism helps you identify risk factors in assemblies where moisture can infiltrate tight interfaces, enhancing preventive maintenance strategies.
Defining Galvanic Corrosion: Process and Causes
Galvanic corrosion occurs when two dissimilar metals are electrically connected in the presence of an electrolyte, causing one metal (the anode) to corrode faster than it would alone while the other (the cathode) is protected. This electrochemical process is driven by the difference in electrode potentials between the metals, leading to the flow of ions and electron transfer. Understanding this mechanism helps you select compatible metals and prevent accelerated degradation in your engineering and design applications.
Key Differences Between Wicking and Galvanic Corrosion
Wicking corrosion occurs when moisture and corrosive agents travel through microscopic gaps or capillaries between metal surfaces, causing localized damage without direct electrical contact. Galvanic corrosion arises from electrochemical reactions between two dissimilar metals in electrical contact within an electrolyte, leading to accelerated deterioration of the more anodic metal. Understanding these key differences helps you select appropriate materials and protective measures to prevent structural damage in various environments.
Common Environments Where Wicking Corrosion Occurs
Wicking corrosion commonly occurs in environments where moisture or electrolytes seep into tight gaps or crevices between metal components, such as under gaskets, washers, and insulating materials. These confined spaces often exist in marine, automotive, and industrial settings where humidity, salt spray, or chemical exposure facilitates the capillary action driving corrosion inside joints. Your equipment in these environments requires careful design and material selection to prevent localized degradation caused by trapped corrosive agents.
Typical Applications Vulnerable to Galvanic Corrosion
Galvanic corrosion commonly affects systems where different metals are in electrical contact within a conductive environment, such as in marine structures, pipelines, and HVAC systems. You will find this type of corrosion especially vulnerable in dissimilar metal joints, fasteners, and bimetallic assemblies subjected to moisture or electrolytes. Understanding the typical applications at risk for galvanic corrosion is critical for selecting compatible materials and protective measures.
Materials Most Susceptible to Wicking vs. Galvanic Corrosion
Materials most susceptible to wicking corrosion include dissimilar metals with microscopic gaps or cracks, such as aluminum alloys paired with steel or copper, where moisture transport causes localized deterioration. Galvanic corrosion primarily affects metals with significant electrochemical potential differences, like zinc coupled with copper or stainless steel, accelerating metal loss at the anodic site. Your choice of materials in multi-metal assemblies should consider these susceptibilities to prevent structural failure and extend service life.
Visual Signs and Detection Methods for Both Corrosion Types
Wicking corrosion typically appears as dark, discolored streaks or localized pitting near bolted or threaded joints where moisture has wicked into crevices, while galvanic corrosion is characterized by corrosion deposits or metal loss primarily at the junction between two dissimilar metals. Visual inspection using magnification, coupled with methods such as dye penetrant testing and microscopy, helps detect the subtle signs of wicking corrosion, whereas galvanic corrosion is often identified through surface potential measurements and ultrasonic thickness gauging. Understanding these visual signs and utilizing targeted detection methods enables you to accurately diagnose and differentiate between wicking and galvanic corrosion for effective maintenance.
Prevention Strategies for Wicking and Galvanic Corrosion
Effective prevention strategies for wicking corrosion include using sealants and coatings to block moisture ingress and selecting materials with compatible thermal expansion properties to avoid micro-gaps. Galvanic corrosion prevention involves electrically isolating dissimilar metals through insulating materials, applying protective coatings, and designing systems to minimize electrolyte accumulation between metal junctions. Both corrosion types benefit from regular maintenance and environmental control to reduce exposure to corrosive agents and moisture.
Impact of Corrosion Type on Reliability and Longevity
Wicking corrosion often leads to localized damage that can compromise the integrity of microelectronic connections, reducing reliability and shortening device lifespan. In contrast, galvanic corrosion occurs due to electrochemical reactions between dissimilar metals, causing more widespread material degradation that accelerates failure rates in structural components. Understanding the specific corrosion mechanisms affecting Your systems is crucial for selecting appropriate materials and protective measures to enhance durability and ensure long-term performance.
Summary: Choosing the Right Mitigation Techniques
Wicking corrosion occurs from the capillary action pulling corrosive agents into tiny crevices between dissimilar metals, while galvanic corrosion results from electrochemical reactions between two different metals in contact. Effective mitigation for wicking corrosion involves sealing joints and applying corrosion inhibitors, whereas galvanic corrosion is best addressed by using compatible metals, insulating materials, or sacrificial anodes. Your choice of protection depends on accurately identifying the corrosion type and applying targeted solutions to extend the lifespan of your metal components.
wicking corrosion vs galvanic corrosion Infographic
 libmatt.com
libmatt.com