Distillation extracts essential oils by vaporizing and condensing plant materials, preserving their purity and intensity, while maceration involves soaking plant parts in a solvent to release fragrance and therapeutic compounds slowly. Your choice depends on the desired concentration and method of extraction for herbs or flowers.
Table of Comparison
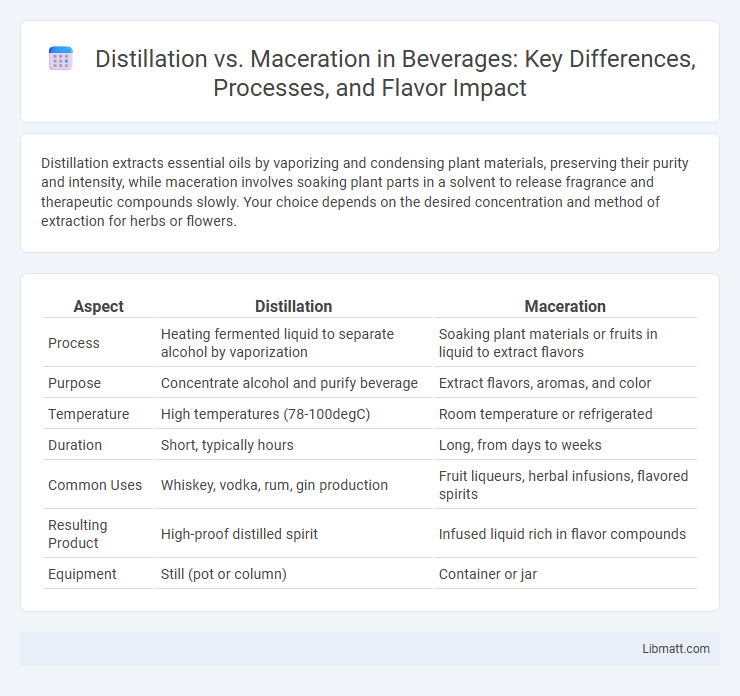
| Aspect | Distillation | Maceration |
|---|---|---|
| Process | Heating fermented liquid to separate alcohol by vaporization | Soaking plant materials or fruits in liquid to extract flavors |
| Purpose | Concentrate alcohol and purify beverage | Extract flavors, aromas, and color |
| Temperature | High temperatures (78-100degC) | Room temperature or refrigerated |
| Duration | Short, typically hours | Long, from days to weeks |
| Common Uses | Whiskey, vodka, rum, gin production | Fruit liqueurs, herbal infusions, flavored spirits |
| Resulting Product | High-proof distilled spirit | Infused liquid rich in flavor compounds |
| Equipment | Still (pot or column) | Container or jar |
Introduction to Distillation and Maceration
Distillation and maceration are two essential extraction methods used in fragrance and essential oil production. Distillation involves heating plant materials to vaporize and then condense volatile compounds, preserving the essence with high purity. Maceration extracts aromas by soaking raw materials in a solvent over time, capturing a broader range of aromatic notes ideal for your personalized scent creations.
Definition of Distillation
Distillation is a process that extracts essential oils and aromatic compounds by heating plant material to produce vapor, which is then condensed into liquid form. Unlike maceration, which involves soaking plant material in a solvent to release flavors and scents, distillation uses steam or water to separate volatile components based on their boiling points. Your choice between distillation and maceration depends on the desired purity and complexity of the extracted essence.
Definition of Maceration
Maceration is a process where plant materials are soaked in a solvent, typically alcohol or oil, at room temperature to extract essential oils, aromas, and active compounds. Unlike distillation, which uses heat and vaporization to separate components, maceration relies on prolonged soaking to release the desired substances. This method preserves delicate, heat-sensitive compounds that might degrade during steam or water distillation.
Key Differences Between Distillation and Maceration
Distillation separates components based on boiling points by heating the material to vaporize volatile compounds, then condensing the vapor to collect essential oils or purified liquids. Maceration involves soaking plant materials in a solvent, typically oil or alcohol, to extract aromatic compounds and flavors without heat application. Distillation is ideal for capturing volatile, heat-stable compounds, whereas maceration is suited for extracting delicate, heat-sensitive substances or non-volatile oils.
How Distillation Works
Distillation works by heating a liquid mixture to create vapor and then cooling the vapor to collect the condensed liquid, effectively separating components based on their boiling points. In essential oil extraction, steam distillation allows volatile aromatic compounds to evaporate and be captured, preserving their purity and concentration. Your choice between distillation and maceration depends on the desired intensity and chemical profile of the extracted essence.
How Maceration Works
Maceration involves soaking plant materials in a liquid, usually oil or alcohol, to extract essential oils, flavors, or bioactive compounds through gentle heat or room temperature over time. This process breaks down cell walls, releasing aromatic compounds without applying intense heat, preserving delicate fragrances and therapeutic properties. Your choice of solvent and duration directly influences the potency and quality of the macerated extract.
Applications in the Food and Beverage Industry
Distillation is widely used in the food and beverage industry for producing spirits such as whiskey, vodka, and rum by separating alcohol from fermented mixtures, ensuring high purity and concentrated flavors. Maceration is employed to extract flavors, colors, and aromatic compounds from fruits, herbs, and spices into liquids like liqueurs, infused oils, and fruit syrups. Both processes enhance the sensory profiles of products, with distillation offering precise alcohol content control while maceration emphasizes natural, robust infusions.
Pros and Cons of Distillation
Distillation offers high purity and concentration of essential oils by using controlled heat to separate volatile compounds, making it ideal for capturing aromatic extracts with minimal contamination. However, the process can be energy-intensive and may degrade heat-sensitive components, resulting in altered or reduced therapeutic properties. Distillation is best suited for plants with robust oils, while delicate flowers are often better processed through maceration to preserve their full chemical profile.
Pros and Cons of Maceration
Maceration preserves delicate aromas and flavors by soaking plant materials in a solvent without heat, ensuring a richer and more authentic extract, though it requires longer processing times compared to distillation. The method is cost-effective and simple but can risk microbial contamination and yields lower purity levels than distillation. Maceration is ideal for extracting heat-sensitive compounds but may result in less concentrated essential oils.
Choosing the Right Extraction Method
Distillation excels in extracting volatile compounds and essential oils with high purity, ideal for creating concentrated aromas or flavors. Maceration, involving soaking plant material in a solvent, preserves delicate compounds and is preferred for producing tinctures or herbal extracts with complex chemical profiles. Selecting the right extraction method depends on the desired final product's chemical stability, potency, and intended use.
Distillation vs maceration Infographic
 libmatt.com
libmatt.com