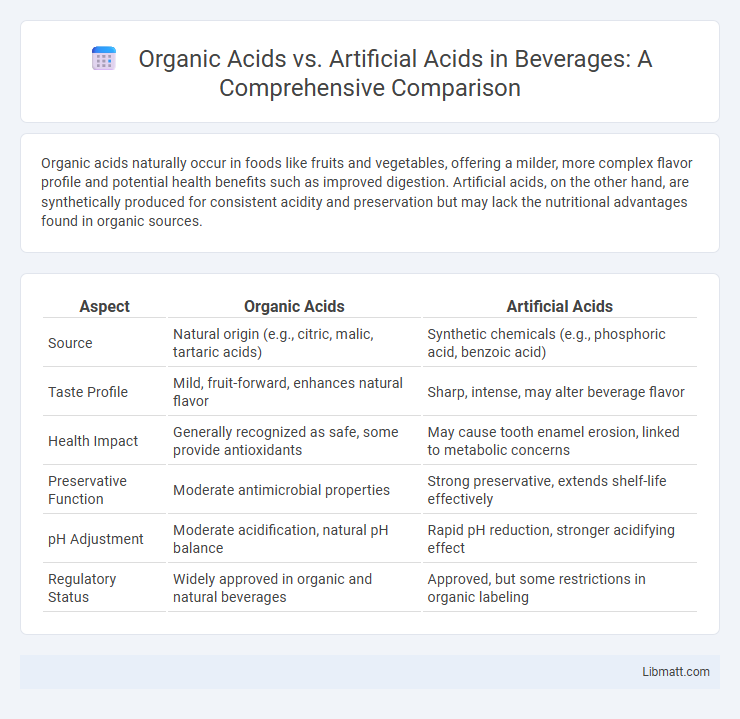
Organic acids naturally occur in foods like fruits and vegetables, offering a milder, more complex flavor profile and potential health benefits such as improved digestion. Artificial acids, on the other hand, are synthetically produced for consistent acidity and preservation but may lack the nutritional advantages found in organic sources.
Table of Comparison
| Aspect | Organic Acids | Artificial Acids |
|---|---|---|
| Source | Natural origin (e.g., citric, malic, tartaric acids) | Synthetic chemicals (e.g., phosphoric acid, benzoic acid) |
| Taste Profile | Mild, fruit-forward, enhances natural flavor | Sharp, intense, may alter beverage flavor |
| Health Impact | Generally recognized as safe, some provide antioxidants | May cause tooth enamel erosion, linked to metabolic concerns |
| Preservative Function | Moderate antimicrobial properties | Strong preservative, extends shelf-life effectively |
| pH Adjustment | Moderate acidification, natural pH balance | Rapid pH reduction, stronger acidifying effect |
| Regulatory Status | Widely approved in organic and natural beverages | Approved, but some restrictions in organic labeling |
Understanding Organic Acids: Definition and Sources
Organic acids are naturally occurring compounds characterized by the presence of one or more carboxyl groups (-COOH), commonly found in fruits, vegetables, and fermented products such as citric acid in citrus fruits and lactic acid in yogurt. These acids play essential roles in metabolic processes and contribute to the flavor, preservation, and nutritional value of natural foods. Unlike artificial acids, which are synthetically produced for industrial and food additive purposes, organic acids derive directly from biological sources.
What Are Artificial Acids? Key Characteristics
Artificial acids are synthetic compounds designed to mimic the sourness and preservative qualities of natural organic acids. These acids, such as citric acid or ascorbic acid produced through industrial processes, offer consistent potency and longer shelf life compared to their organic counterparts. Understanding the key characteristics of artificial acids helps you choose the right acidulant for food preservation, flavor enhancement, or industrial applications.
Chemical Structure: Organic vs Artificial Acids
Organic acids contain carbon atoms bonded to hydrogen and functional groups like carboxyl (-COOH), which give them natural acid properties found in fruits and biological systems. Artificial acids, often synthetic chemicals such as sulfuric or hydrochloric acid, lack carbon-hydrogen frameworks and consist primarily of inorganic elements resulting in stronger corrosive behavior. Understanding these differences in chemical structures helps you choose acids suitable for applications requiring natural origin or synthetic potency.
Occurrence in Nature and Industry
Organic acids, such as citric acid and acetic acid, naturally occur in fruits, vegetables, and fermented products, playing key roles in metabolism and preservation. Artificial acids, including synthetic sulfuric acid and hydrochloric acid, are predominantly produced in industrial settings for applications in manufacturing, food processing, and pharmaceuticals. The natural availability of organic acids contrasts with the large-scale chemical synthesis and controlled purity of artificial acids used in various industrial processes.
Health Impacts: Benefits and Risks
Organic acids, such as citric acid from fruits, offer natural antioxidant properties and support digestion, contributing to improved gut health and reduced inflammation. Artificial acids, including synthetic citric or phosphoric acid commonly found in processed foods and sodas, may cause enamel erosion and increase the risk of acid reflux or digestive discomfort when consumed excessively. Your choice between organic and artificial acids can influence long-term health outcomes by balancing natural benefits with potential risks linked to synthetic additives.
Role in Food Preservation and Flavoring
Organic acids such as citric acid, lactic acid, and acetic acid play a crucial role in food preservation by lowering pH levels, thereby inhibiting microbial growth and extending shelf life. These acids also contribute to natural flavor profiles, enhancing the tartness and freshness of fruits, dairy, and fermented products. Artificial acids, including synthetic versions of these compounds, provide consistent acidity and flavor control but may lack the complexity and health benefits associated with natural organic acids.
Environmental Effects of Acid Use
Organic acids, derived from natural sources like fruits and plants, typically biodegrade faster and present lower toxicity risks to ecosystems compared to artificial acids such as sulfuric or hydrochloric acid. Artificial acids often contribute to soil acidification, water pollution, and harm aquatic life due to their strong corrosive properties and persistence in the environment. Choosing organic acids can reduce environmental footprint by minimizing harmful residues and supporting sustainable agricultural and industrial practices.
Safety and Toxicity Comparisons
Organic acids such as citric acid and lactic acid generally exhibit lower toxicity and better safety profiles compared to artificial acids like hydrochloric acid or sulfuric acid, which are often corrosive and pose significant health risks upon exposure. Your consumption or use of organic acids in food and cosmetic products is typically safer due to their natural origin and biodegradability, reducing potential for adverse effects. Regulatory agencies often classify organic acids as GRAS (Generally Recognized As Safe), while artificial acids require stricter handling and usage guidelines to mitigate toxicity concerns.
Applications in Everyday Products
Organic acids such as citric acid and acetic acid are widely used in food preservation, flavoring, and skincare products due to their natural origin and biodegradability. Artificial acids like sulfuric acid and phosphoric acid find applications in industrial cleaning agents, fertilizers, and soft drinks for pH adjustment and preservation. The choice between organic and artificial acids depends on the specific product requirements, safety considerations, and environmental impact.
Choosing Between Organic and Artificial Acids: Factors to Consider
When choosing between organic and artificial acids, consider factors such as source safety, environmental impact, and intended application. Organic acids, derived from natural sources like fruits or fermentation, offer biodegradability and lower toxicity, making them suitable for food and cosmetic products. Artificial acids typically provide more consistent concentration and cost-effectiveness, which may be ideal for industrial processes requiring precise acidity control, aligning your choice with your product's safety standards and performance requirements.
Organic acids vs artificial acids Infographic
 libmatt.com
libmatt.com