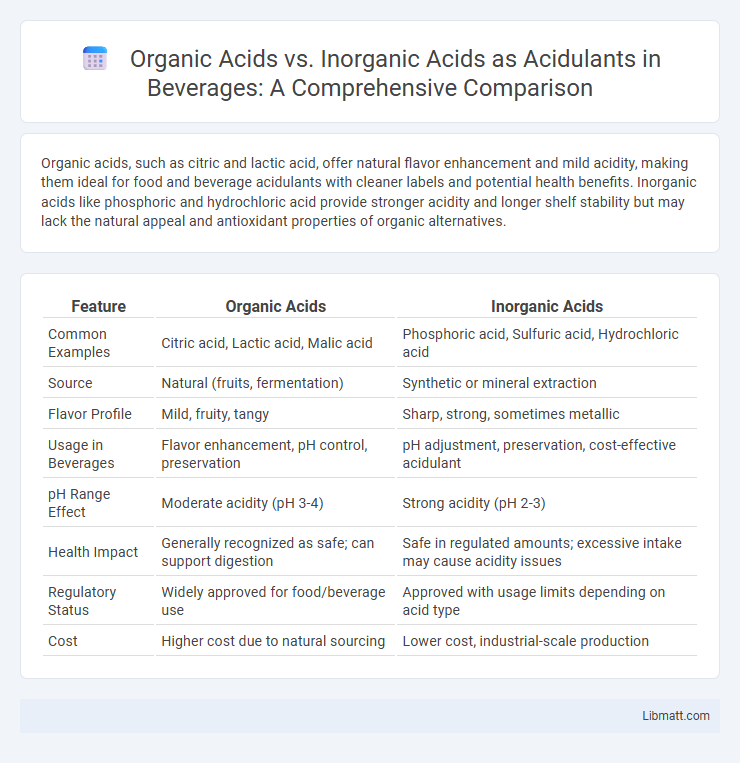
Organic acids, such as citric and lactic acid, offer natural flavor enhancement and mild acidity, making them ideal for food and beverage acidulants with cleaner labels and potential health benefits. Inorganic acids like phosphoric and hydrochloric acid provide stronger acidity and longer shelf stability but may lack the natural appeal and antioxidant properties of organic alternatives.
Table of Comparison
| Feature | Organic Acids | Inorganic Acids |
|---|---|---|
| Common Examples | Citric acid, Lactic acid, Malic acid | Phosphoric acid, Sulfuric acid, Hydrochloric acid |
| Source | Natural (fruits, fermentation) | Synthetic or mineral extraction |
| Flavor Profile | Mild, fruity, tangy | Sharp, strong, sometimes metallic |
| Usage in Beverages | Flavor enhancement, pH control, preservation | pH adjustment, preservation, cost-effective acidulant |
| pH Range Effect | Moderate acidity (pH 3-4) | Strong acidity (pH 2-3) |
| Health Impact | Generally recognized as safe; can support digestion | Safe in regulated amounts; excessive intake may cause acidity issues |
| Regulatory Status | Widely approved for food/beverage use | Approved with usage limits depending on acid type |
| Cost | Higher cost due to natural sourcing | Lower cost, industrial-scale production |
Introduction to Acidulants in Food and Beverage Industry
Acidulants play a crucial role in the food and beverage industry by enhancing flavor, preserving freshness, and controlling pH levels. Organic acids, such as citric acid and lactic acid, are naturally derived and often preferred for their mild taste and health benefits. Inorganic acids like phosphoric acid provide strong acidity and longer shelf stability, making them ideal for carbonated beverages and processed foods.
Defining Organic Acids and Inorganic Acids
Organic acids are carbon-based compounds containing carboxyl groups, commonly found in natural sources like fruits and fermented products, and include acids such as citric, lactic, and acetic acid. Inorganic acids, also known as mineral acids, are non-carbon-containing acids like hydrochloric, sulfuric, and phosphoric acid, typically derived from mineral sources. These acids differ in their chemical structure, origin, and applications in food acidulants, where organic acids often provide flavor and preservation benefits, while inorganic acids primarily function as strong acidulants and pH regulators.
Chemical Properties and Structures
Organic acids as acidulants typically contain carboxyl groups (-COOH) attached to carbon-based chains, exhibiting weak acidity due to partial dissociation in aqueous solutions, examples include citric acid and lactic acid with molecular structures allowing hydrogen bonding and chelation. Inorganic acids, such as hydrochloric acid and sulfuric acid, consist of hydrogen ions bonded to non-carbon elements and dissociate completely in water, resulting in strong acidity and a simpler molecular structure without carbon backbones. The presence of carbon atoms in organic acids influences their chemical reactivity, solubility, and flavor profiles, whereas inorganic acids provide higher proton availability and stronger acidity useful for pH control.
Common Types of Organic Acidulants
Common types of organic acidulants include citric acid, lactic acid, malic acid, and acetic acid, which are widely used in food and beverage industries for flavor enhancement and pH adjustment. Citric acid, derived from citrus fruits, is particularly effective for its strong sour taste and natural preservative properties. Lactic acid, produced through fermentation, and malic acid, found in apples, contribute to mild acidity and freshness, while acetic acid, the main component of vinegar, offers a distinctive sharpness in acidulation.
Common Types of Inorganic Acidulants
Common types of inorganic acidulants include hydrochloric acid, sulfuric acid, and phosphoric acid, widely used for their strong acidifying properties in food preservation and flavor enhancement. Phosphoric acid is particularly prevalent in beverages like colas, providing a tangy taste and microbial inhibition. Hydrochloric and sulfuric acids are more common in industrial applications but can be utilized under controlled conditions in food processing for pH regulation and acidification.
Functional Roles in Food Applications
Organic acids such as citric, lactic, and malic acids play crucial roles in food applications by enhancing flavor, acting as natural preservatives, and maintaining product stability. Inorganic acids like phosphoric and sulfuric acids primarily function as acidulants to adjust pH levels, improve textures, and inhibit microbial growth in processed foods. Your choice between organic and inorganic acids depends on desired sensory properties, shelf life, and regulatory standards in food formulation.
Impact on Flavor and Sensory Profiles
Organic acids, such as citric and malic acid, impart a natural, fruity tartness that enhances the freshness and complexity of flavor profiles, making them ideal for beverages and fruit-based products. Inorganic acids, like phosphoric and sulfuric acid, provide a sharper, more acidic taste that can intensify sourness but may lack the nuanced sensory appeal of organic acids. Your choice of acidulant significantly influences the overall sensory experience, balancing acidity with flavor depth and mouthfeel.
Safety, Toxicity, and Regulatory Perspectives
Organic acids such as citric acid and lactic acid are generally recognized as safe (GRAS) by regulatory agencies like the FDA due to their natural occurrence and low toxicity profiles, making them favorable acidulants in food and pharmaceutical products. Inorganic acids, including phosphoric acid and sulfuric acid, exhibit higher corrosiveness and toxicity risks, necessitating stringent regulatory controls and specific usage limits to ensure consumer safety. Regulatory frameworks often mandate detailed safety assessments and maximum allowable concentrations for both acid types, prioritizing human health and environmental impact during product formulation and approval processes.
Sustainability and Environmental Considerations
Organic acids, such as citric and lactic acid, are derived from renewable resources and typically biodegrade more easily, reducing environmental impact compared to many inorganic acids like sulfuric or hydrochloric acid. Their production often involves fermentation processes that utilize agricultural byproducts, enhancing sustainability through waste valorization. Inorganic acids, while effective as acidulants, usually rely on mineral extraction with higher energy consumption and potential ecological risks from mining and chemical waste.
Selecting the Right Acidulant: Factors and Recommendations
Selecting the right acidulant between organic acids like citric, malic, and lactic acids and inorganic acids such as phosphoric and sulfuric acids depends on factors including desired pH, flavor profile, solubility, and food application. Organic acids are preferred in beverages and dairy for their mild taste and natural origin, while inorganic acids are effective in processed foods requiring strong acidity and mineral fortification. Recommendations emphasize balancing acid strength, sensory impact, regulatory compliance, and compatibility with other ingredients to optimize product quality and stability.
Organic acids vs inorganic acids (as acidulants) Infographic
 libmatt.com
libmatt.com