Sucrose, a disaccharide composed of glucose and fructose, provides a sweet, crystalline texture ideal for baking and candy-making, while glucose syrup, a liquid sweetener derived from starch, offers enhanced moisture retention and prevents crystallization, improving texture and shelf life in processed foods. Understanding these differences helps you choose the right sweetener for your culinary or manufacturing needs.
Table of Comparison
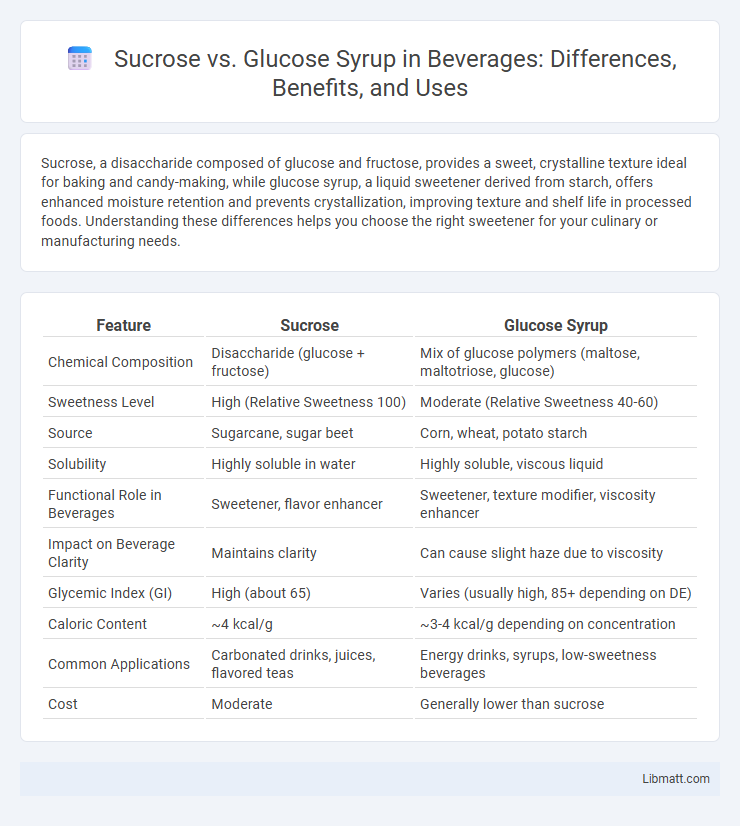
| Feature | Sucrose | Glucose Syrup |
|---|---|---|
| Chemical Composition | Disaccharide (glucose + fructose) | Mix of glucose polymers (maltose, maltotriose, glucose) |
| Sweetness Level | High (Relative Sweetness 100) | Moderate (Relative Sweetness 40-60) |
| Source | Sugarcane, sugar beet | Corn, wheat, potato starch |
| Solubility | Highly soluble in water | Highly soluble, viscous liquid |
| Functional Role in Beverages | Sweetener, flavor enhancer | Sweetener, texture modifier, viscosity enhancer |
| Impact on Beverage Clarity | Maintains clarity | Can cause slight haze due to viscosity |
| Glycemic Index (GI) | High (about 65) | Varies (usually high, 85+ depending on DE) |
| Caloric Content | ~4 kcal/g | ~3-4 kcal/g depending on concentration |
| Common Applications | Carbonated drinks, juices, flavored teas | Energy drinks, syrups, low-sweetness beverages |
| Cost | Moderate | Generally lower than sucrose |
Introduction to Sucrose and Glucose Syrup
Sucrose, commonly known as table sugar, is a disaccharide composed of glucose and fructose molecules, widely used as a sweetening agent in food products. Glucose syrup, derived from starch hydrolysis, consists primarily of glucose molecules and varies in sweetness and viscosity depending on its dextrose equivalent (DE) value. Both sucrose and glucose syrup serve distinct functional roles in confectionery, baking, and food manufacturing due to their different chemical structures and physical properties.
Chemical Composition and Structure
Sucrose is a disaccharide composed of one glucose and one fructose molecule linked by a glycosidic bond, whereas glucose syrup primarily contains glucose polymers such as maltose and maltotriose, derived from starch hydrolysis. The chemical structure of sucrose includes a-D-glucopyranosyl and b-D-fructofuranosyl units, making it a non-reducing sugar, while glucose syrup contains reducing sugars with free aldehyde groups. These structural differences impact their sweetness, solubility, and functional properties in food applications.
Sources and Production Methods
Sucrose, commonly derived from sugarcane and sugar beets, undergoes a refining process that extracts crystalline sugar through evaporation and centrifugation. Glucose syrup is produced by enzymatic hydrolysis of starch sources such as corn, wheat, or potatoes, breaking down starch into glucose molecules. The production of glucose syrup involves liquefaction and saccharification steps using enzymes like alpha-amylase and glucoamylase to convert starch into fermentable sugars.
Taste and Sweetness Comparison
Sucrose, commonly known as table sugar, provides a clean, sweet taste with a high sweetness level, approximately 1.0 on the relative sweetness scale. Glucose syrup, derived from hydrolyzed starch, offers a milder sweetness, ranging from 0.7 to 0.8, with a less pronounced sugary flavor and a slightly viscous texture that can affect mouthfeel. The subtle sweetness and functional properties of glucose syrup make it ideal for mellowing flavor intensity and improving texture in confectionery and baked goods.
Functional Uses in Food Industry
Sucrose serves as a primary sweetener and crystallization agent in confectionery, baking, and beverages, providing texture and enhancing flavor. Glucose syrup offers superior moisture retention, viscosity control, and non-crystallizing properties, making it essential for chewy candies, ice cream, and baked goods. Your product formulations benefit from choosing sucrose for sweetness and crystalline structure, while glucose syrup ensures smoothness and extended shelf life.
Nutritional Value and Caloric Content
Sucrose and glucose syrup differ significantly in nutritional value and caloric content, with sucrose providing about 4 calories per gram derived from a disaccharide composed of glucose and fructose, while glucose syrup contains primarily glucose molecules and varies in caloric density depending on concentration but generally remains close to 3.4 calories per gram. Sucrose impacts blood sugar levels moderately due to fructose content, whereas glucose syrup causes a quicker spike in blood glucose because it is rapidly absorbed. Your choice between these sweeteners can influence energy metabolism and glycemic response, important factors in dietary planning and managing conditions like diabetes.
Effects on Blood Sugar Levels
Sucrose is a disaccharide composed of glucose and fructose, causing a moderate rise in blood sugar levels as it is broken down during digestion. Glucose syrup contains primarily glucose, leading to a more rapid and higher spike in blood glucose due to its simpler sugar structure. Both impact insulin response, but glucose syrup generally results in quicker and more pronounced effects on blood sugar control.
Health Implications and Risks
Sucrose and glucose syrup vary significantly in their health implications; sucrose, a disaccharide composed of glucose and fructose, can contribute to insulin resistance and increased risk of metabolic disorders when consumed excessively. Glucose syrup, primarily consisting of glucose, is rapidly absorbed, leading to quick spikes in blood sugar levels and potential insulin imbalances. Both sweeteners are linked to obesity, dental caries, and increased risk of type 2 diabetes when intake exceeds recommended dietary guidelines.
Labeling and Identification in Products
Sucrose and glucose syrup are labeled differently due to their distinct chemical compositions, with sucrose commonly identified as cane sugar or beet sugar, and glucose syrup often listed as glucose-fructose syrup or corn syrup. Product labels must comply with regulatory standards such as those from the FDA or EFSA, ensuring accurate ingredient disclosure to prevent consumer confusion, especially in terms of allergen and dietary concerns. Clear identification assists in distinguishing sucrose's disaccharide structure from glucose syrup's mixture of glucose and other saccharides, which impacts product usage and nutritional labeling.
Environmental Impact of Production
Sucrose production from sugarcane or sugar beets typically involves extensive land use, water consumption, and agrochemical inputs, contributing to deforestation and soil degradation. Glucose syrup, often derived from corn via enzymatic hydrolysis, is associated with monoculture farming practices that increase pesticide use and water depletion, particularly in major corn-producing regions like the United States. Both processes have carbon footprints linked to cultivation, processing, and transportation, but glucose syrup's industrial refinement often results in higher energy consumption compared to traditional sucrose extraction methods.
Sucrose vs glucose syrup Infographic
 libmatt.com
libmatt.com