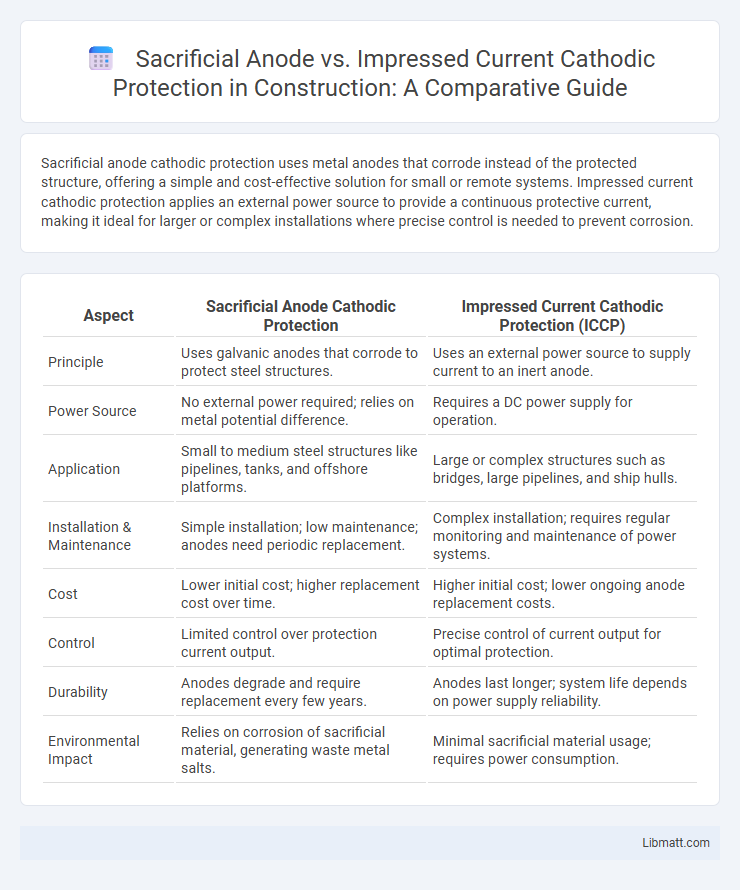
Sacrificial anode cathodic protection uses metal anodes that corrode instead of the protected structure, offering a simple and cost-effective solution for small or remote systems. Impressed current cathodic protection applies an external power source to provide a continuous protective current, making it ideal for larger or complex installations where precise control is needed to prevent corrosion.
Table of Comparison
| Aspect | Sacrificial Anode Cathodic Protection | Impressed Current Cathodic Protection (ICCP) |
|---|---|---|
| Principle | Uses galvanic anodes that corrode to protect steel structures. | Uses an external power source to supply current to an inert anode. |
| Power Source | No external power required; relies on metal potential difference. | Requires a DC power supply for operation. |
| Application | Small to medium steel structures like pipelines, tanks, and offshore platforms. | Large or complex structures such as bridges, large pipelines, and ship hulls. |
| Installation & Maintenance | Simple installation; low maintenance; anodes need periodic replacement. | Complex installation; requires regular monitoring and maintenance of power systems. |
| Cost | Lower initial cost; higher replacement cost over time. | Higher initial cost; lower ongoing anode replacement costs. |
| Control | Limited control over protection current output. | Precise control of current output for optimal protection. |
| Durability | Anodes degrade and require replacement every few years. | Anodes last longer; system life depends on power supply reliability. |
| Environmental Impact | Relies on corrosion of sacrificial material, generating waste metal salts. | Minimal sacrificial material usage; requires power consumption. |
Introduction to Cathodic Protection Methods
Cathodic protection methods prevent corrosion on metal structures by converting the entire surface into a cathode through electrochemical means. Sacrificial anode systems use galvanic metals such as zinc, magnesium, or aluminum, which corrode preferentially to protect the main structure without external power. Impressed current cathodic protection employs an external DC power source to drive current through inert anodes like graphite or platinum, offering precise control and suitability for larger or more challenging environments.
Overview of Sacrificial Anode Systems
Sacrificial anode systems use galvanic corrosion protection by attaching more active metal anodes, such as zinc, magnesium, or aluminum, to the structure to be protected, which corrodes instead of the metal surface. These systems require no external power source, relying on the electrochemical potential difference between the anode and cathode to prevent corrosion on pipelines, tanks, and marine vessels. Sacrificial anodes are ideal for smaller or less complex structures and environments with lower current demand, offering a simple, maintenance-minimal corrosion protection method.
Impressed Current Cathodic Protection Explained
Impressed Current Cathodic Protection (ICCP) uses a power source to deliver a continuous flow of protective current to metal structures, preventing corrosion by making the metal a cathode in an electrochemical cell. Unlike sacrificial anodes which consume themselves to protect the metal, ICCP systems rely on inert anodes and an external DC power supply to maintain protection over long distances and complex structures like pipelines or ship hulls. Your infrastructure benefits from ICCP when consistent, adjustable corrosion control is needed, especially in environments where sacrificial anode capacity may be insufficient.
Key Components of Each System
Sacrificial anode cathodic protection systems consist primarily of galvanic anodes made from metals like zinc, magnesium, or aluminum, which corrode sacrificially to protect the steel structure. Impressed current cathodic protection (ICCP) systems include an external power source, inert anodes such as titanium mesh or graphite, and a control panel that regulates current output to prevent corrosion. Both systems require electrical isolation and reference electrodes to monitor protection levels and ensure optimal performance.
How Sacrificial Anodes Prevent Corrosion
Sacrificial anodes prevent corrosion by acting as a more reactive metal that corrodes instead of the protected structure, effectively serving as a sacrificial metal in cathodic protection systems. Common materials like zinc, magnesium, or aluminum anodes undergo oxidation, thereby preserving the integrity of your pipelines, tanks, or marine vessels. This electrochemical process ensures that the protected metal remains cathodic, significantly reducing corrosion rates and extending the lifespan of your assets.
Mechanism of Impressed Current Systems
Impressed current cathodic protection (ICCP) systems operate by applying an external direct current to the structure through an inert anode, effectively counteracting corrosion by making the metal surface a cathode. The system uses a DC power source to deliver current that flows from the anode to the protected structure, ensuring a continuous and controlled protective electric potential. This controlled current flow prevents the oxidation reaction that causes metal deterioration, extending the lifespan of pipelines, tanks, and marine structures.
Installation and Maintenance Requirements
Sacrificial anode systems require straightforward installation with anodes made of zinc, magnesium, or aluminum attached directly to the metal structure, typically needing minimal ongoing maintenance as the anodes corrode and must be replaced periodically. Impressed current cathodic protection (ICCP) systems involve more complex installation, including a power source, inert anodes, and control panels, with regular maintenance required to monitor electrical output, inspect system components, and replace power supplies as needed. Your choice between the two depends on factors such as the structure's size, environment, and long-term maintenance capabilities.
Cost Comparison: Initial and Long-Term Expenses
Sacrificial anode systems generally have lower initial costs due to simpler installation and fewer components, making them cost-effective for smaller-scale structures or less aggressive environments. Impressed current cathodic protection (ICCP) involves higher upfront expenses for power supplies and control equipment but offers lower long-term maintenance costs and better performance for large or complex infrastructures. Your choice should balance initial budget constraints against ongoing operational costs, factoring in the expected service life and environmental conditions of the protected asset.
Performance and Suitability in Different Environments
Sacrificial anode systems provide reliable corrosion protection by using metal alloys like zinc or magnesium that corrode preferentially, making them ideal for environments with limited electrical infrastructure or small-scale applications. Impressed current cathodic protection (ICCP) delivers superior performance in large-scale or highly corrosive environments, offering adjustable current output to maintain optimal protection levels even under fluctuating conditions. Your choice between these methods depends on factors such as environmental aggressiveness, structure size, and maintenance capabilities, with ICCP favored for long-term control and sacrificial anodes suited for simplicity and ease of use.
Choosing the Right Cathodic Protection for Your Application
Selecting the ideal cathodic protection depends on factors such as structure size, environmental conditions, and maintenance capabilities. Sacrificial anodes offer a simple, low-maintenance solution for small structures in less aggressive environments, while impressed current systems provide greater control and are suited for larger or highly corrosive settings. Evaluating metal type, soil resistivity, and lifecycle costs ensures the chosen method delivers effective corrosion prevention tailored to specific operational needs.
Sacrificial anode vs impressed current cathodic protection Infographic
 libmatt.com
libmatt.com