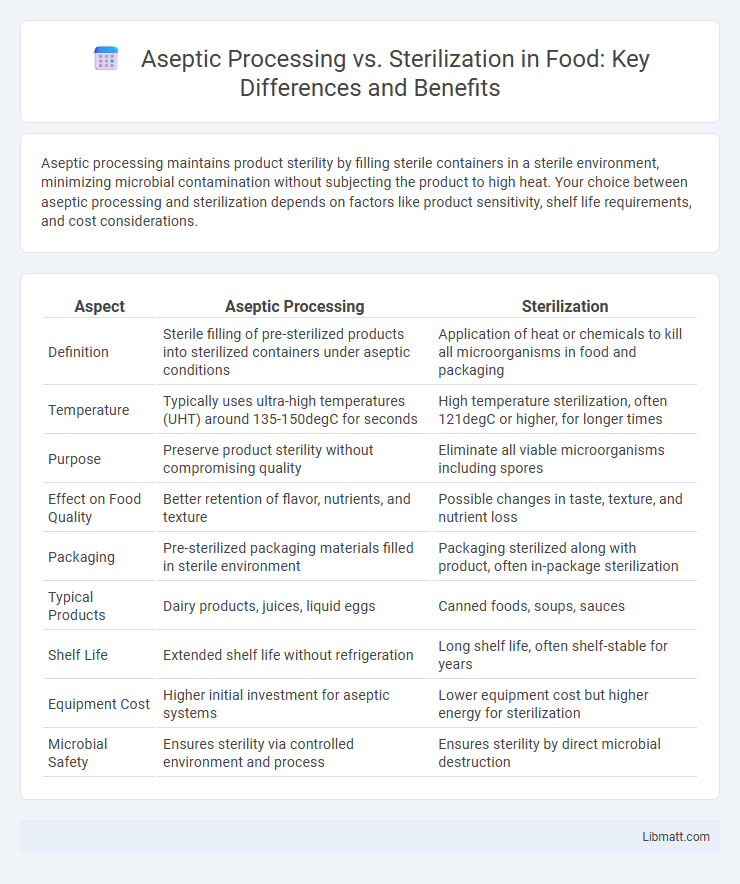
Aseptic processing maintains product sterility by filling sterile containers in a sterile environment, minimizing microbial contamination without subjecting the product to high heat. Your choice between aseptic processing and sterilization depends on factors like product sensitivity, shelf life requirements, and cost considerations.
Table of Comparison
| Aspect | Aseptic Processing | Sterilization |
|---|---|---|
| Definition | Sterile filling of pre-sterilized products into sterilized containers under aseptic conditions | Application of heat or chemicals to kill all microorganisms in food and packaging |
| Temperature | Typically uses ultra-high temperatures (UHT) around 135-150degC for seconds | High temperature sterilization, often 121degC or higher, for longer times |
| Purpose | Preserve product sterility without compromising quality | Eliminate all viable microorganisms including spores |
| Effect on Food Quality | Better retention of flavor, nutrients, and texture | Possible changes in taste, texture, and nutrient loss |
| Packaging | Pre-sterilized packaging materials filled in sterile environment | Packaging sterilized along with product, often in-package sterilization |
| Typical Products | Dairy products, juices, liquid eggs | Canned foods, soups, sauces |
| Shelf Life | Extended shelf life without refrigeration | Long shelf life, often shelf-stable for years |
| Equipment Cost | Higher initial investment for aseptic systems | Lower equipment cost but higher energy for sterilization |
| Microbial Safety | Ensures sterility via controlled environment and process | Ensures sterility by direct microbial destruction |
Introduction to Aseptic Processing and Sterilization
Aseptic processing involves sterilizing a product and its packaging separately before combining them in a sterile environment to prevent contamination. Sterilization, on the other hand, refers to the direct application of physical or chemical methods such as heat, radiation, or filtration to eliminate all microorganisms in the final product. Your choice between aseptic processing and sterilization depends on factors like product type, shelf-life requirements, and sensitivity to heat or chemicals.
Definitions: Aseptic Processing vs Sterilization
Aseptic processing involves the sterile handling and packaging of pre-sterilized components to maintain product sterility without exposing it to contaminants. Sterilization refers to the complete elimination of all microorganisms from a product or surface through physical or chemical methods such as heat, radiation, or gas. Your choice between aseptic processing and sterilization depends on the product's heat sensitivity and the required sterility assurance level.
Key Principles of Aseptic Processing
Aseptic processing relies on maintaining a sterile environment by sterilizing product components separately before assembly, preventing contamination during filling and packaging. This method emphasizes strict environmental controls, validated sterilization techniques, and the use of sterile equipment to ensure product safety and quality. Your understanding of key principles such as sterile filtration, cleanroom standards, and aseptic techniques is essential for effective implementation.
Core Steps in Sterilization Methods
Sterilization methods involve critical core steps such as cleaning, loading, exposure to sterilizing agents (steam, ethylene oxide, or radiation), and post-sterilization handling to maintain sterility. These steps ensure complete microbial inactivation essential for high-risk medical and pharmaceutical products. Your choice between aseptic processing and sterilization depends on product stability and regulatory requirements.
Equipment Used in Aseptic vs Sterile Manufacturing
Aseptic processing employs specialized equipment such as isolators, laminar airflow cabinets, and steam-in-place (SIP) systems to maintain sterility during product filling. In contrast, sterilization manufacturing relies heavily on autoclaves, dry heat sterilizers, and gamma irradiation devices to sterilize equipment and materials before use. Your choice of equipment impacts contamination control, ensuring product safety and compliance with regulatory standards.
Applications in Pharmaceutical and Food Industries
Aseptic processing is widely used in the pharmaceutical industry for manufacturing heat-sensitive products like vaccines and biologics, preserving their potency by avoiding high-temperature sterilization. In the food industry, aseptic processing extends shelf life of products such as dairy and juices by sterilizing packaging and filling in sterile environments. Sterilization methods, including steam autoclaving and gamma irradiation, are essential for terminal sterilization of medical devices and canned foods, ensuring microbial safety through complete eradication of contaminants.
Regulatory Standards and Compliance
Aseptic processing and sterilization both require strict adherence to regulatory standards such as FDA 21 CFR Part 111 and EU GMP guidelines to ensure product safety and efficacy. Aseptic processing demands rigorous environmental monitoring and validation of sterile filtration, whereas sterilization involves validated terminal sterilization methods with precise control of parameters like temperature and exposure time. Compliance with USP <71> for sterility testing and ISO 13485 certification supports risk mitigation and assures consistent quality in both manufacturing processes.
Advantages and Limitations of Each Approach
Aseptic processing offers the advantage of preserving product quality by avoiding high heat exposure, making it ideal for heat-sensitive pharmaceuticals and foods, but it requires stringent sterile conditions to prevent contamination. Sterilization, typically involving heat or radiation, ensures complete microbial destruction, providing a high level of safety, though it may degrade sensitive components and alter product properties. Your choice between these methods depends on balancing the need for microbial control with the preservation of product integrity and regulatory compliance.
Common Challenges and Solutions
Aseptic processing and sterilization both face challenges such as contamination risks, equipment validation, and maintaining product sterility. Common solutions include rigorous environmental monitoring, implementing validated sterilization cycles, and using closed systems to minimize microbial exposure. Your ability to optimize these processes depends on strict adherence to regulatory guidelines and continuous process improvement.
Choosing the Right Method: Factors to Consider
Selecting between aseptic processing and sterilization depends primarily on product heat sensitivity, microbial load, and packaging compatibility. Aseptic processing suits heat-sensitive products requiring sterile environments without altering quality, while sterilization is ideal for highly microbial-prone items tolerating intense heat or chemical agents. Regulatory compliance, cost efficiency, and equipment availability further influence the decision-making process in pharmaceutical and food industries.
Aseptic Processing vs Sterilization Infographic
 libmatt.com
libmatt.com