Prilling forms small, spherical pellets by dropping molten material through a tower where it solidifies in cool air, offering uniform size and shape ideal for fertilizers and chemicals. Spray chilling involves atomizing molten fat into a cold chamber, rapidly solidifying it into fine particles perfect for controlled-release applications in pharmaceuticals and food industries.
Table of Comparison
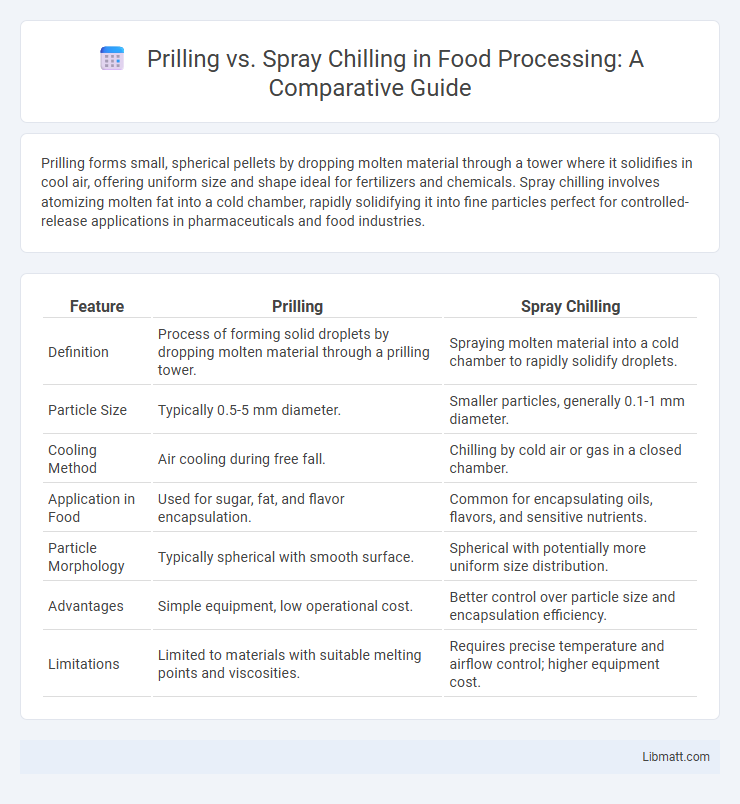
| Feature | Prilling | Spray Chilling |
|---|---|---|
| Definition | Process of forming solid droplets by dropping molten material through a prilling tower. | Spraying molten material into a cold chamber to rapidly solidify droplets. |
| Particle Size | Typically 0.5-5 mm diameter. | Smaller particles, generally 0.1-1 mm diameter. |
| Cooling Method | Air cooling during free fall. | Chilling by cold air or gas in a closed chamber. |
| Application in Food | Used for sugar, fat, and flavor encapsulation. | Common for encapsulating oils, flavors, and sensitive nutrients. |
| Particle Morphology | Typically spherical with smooth surface. | Spherical with potentially more uniform size distribution. |
| Advantages | Simple equipment, low operational cost. | Better control over particle size and encapsulation efficiency. |
| Limitations | Limited to materials with suitable melting points and viscosities. | Requires precise temperature and airflow control; higher equipment cost. |
Introduction to Prilling and Spray Chilling
Prilling is a process where molten material is transformed into spherical granules by dripping it through a prilling tower, allowing rapid solidification in cool air. Spray chilling involves atomizing molten substances into fine droplets that solidify quickly upon contact with a chilled gas or liquid, creating solid particles with controlled size and texture. Your choice between prilling and spray chilling depends on factors such as material properties, desired particle size, and application requirements.
Definition and Process Overview
Prilling is a process where molten materials are atomized into droplets that solidify into spherical granules upon cooling, commonly used for fertilizers and detergents. Spray chilling involves spraying molten fats or waxes into a cooler chamber, causing rapid solidification and encapsulation of active ingredients in a protective lipid matrix. Both techniques transform liquids into solid particles but differ in cooling methods and application industries, with prilling focusing on droplet fall cooling and spray chilling utilizing cold air or gas streams for faster solidification.
Key Differences between Prilling and Spray Chilling
Prilling involves forming solid beads by dropping molten material through a prilling tower where it cools and solidifies, while spray chilling atomizes molten fat or active ingredients into fine droplets that rapidly solidify upon contact with cold air. Prilling typically produces larger, more uniform spherical particles suitable for fertilizers and detergents, whereas spray chilling yields smaller, more controlled particle sizes ideal for encapsulating flavors or nutrients. Your choice depends on desired particle size, application, and thermal sensitivity of the material being processed.
Advantages of Prilling
Prilling offers enhanced particle uniformity and improved flow characteristics compared to spray chilling, making it ideal for consistent dosage in pharmaceuticals and fertilizers. The controlled solidification process in prilling produces spherical granules with high mechanical strength and better thermal stability. This method also enables greater scalability and cost-efficiency in industrial applications due to simpler equipment and faster production rates.
Advantages of Spray Chilling
Spray chilling offers enhanced particle uniformity and improved thermal stability compared to prilling, making it ideal for sensitive and heat-labile substances. This process allows for rapid solidification and finer particle size control, which improves product flowability and dissolution rates. Spray chilling also reduces production time and energy consumption, offering a more efficient encapsulation method in the pharmaceutical and food industries.
Industrial Applications of Prilling
Prilling is widely utilized in industrial applications for producing uniform spherical granules, especially in fertilizers, detergents, and pharmaceuticals, due to its ability to create consistent particle size and improve flow properties. This process involves melting a substance and forming droplets that solidify as they fall through a cooling tower, enhancing product handling and storage efficiency. Your production benefits from prilling's scalability and cost-effectiveness when uniform granules are critical for downstream processing.
Industrial Applications of Spray Chilling
Spray chilling is widely used in the pharmaceutical and food industries for encapsulating active ingredients and flavorings due to its ability to produce uniform, solid lipid particles with controlled release properties. Unlike prilling, spray chilling operates at lower temperatures, preserving heat-sensitive compounds while enabling large-scale production of coated powders and granules. This method enhances the stability and bioavailability of encapsulated materials, making it ideal for nutritional supplements, confectionery coatings, and cosmetic formulations.
Factors Influencing the Choice of Method
Factors influencing the choice between prilling and spray chilling include the physical properties of the active ingredient, such as melting point and thermal sensitivity, which determine the suitability of each method for preserving compound integrity. Particle size distribution and desired release profile also play critical roles, as prilling typically produces larger, more uniform spheres, while spray chilling allows for finer, more controlled particle sizes. Production scale, equipment availability, and process cost further guide the decision, with prilling favored in high-volume applications and spray chilling preferred for heat-sensitive or complex formulations.
Environmental and Economic Considerations
Prilling consumes less energy compared to spray chilling due to lower requirements for temperature and air flow, making it a more environmentally-friendly option in large-scale production. Spray chilling provides superior control over particle size and moisture content, potentially reducing waste and improving product yield, which translates into economic benefits for Your manufacturing process. The choice between prilling and spray chilling impacts both carbon footprint and operational costs, with prilling favoring energy efficiency and spray chilling offering enhanced product quality management.
Future Trends in Particle Formation Technologies
Future trends in particle formation technologies emphasize enhanced control over particle size distribution and morphology through advanced prilling and spray chilling techniques. Innovations in prilling utilize optimized cooling rates and atomization methods to produce uniform microspheres ideal for pharmaceuticals and agrochemicals. Spray chilling advancements focus on temperature modulation and encapsulation efficiency, enabling scalable production of controlled-release particles with improved stability and bioavailability.
prilling vs spray chilling Infographic
 libmatt.com
libmatt.com