Cytotoxic cleanrooms are specifically designed to handle hazardous drugs and chemicals, ensuring stringent contamination control through specialized ventilation and decontamination processes. Your choice between cytotoxic and non-cytotoxic cleanrooms depends on the type of substances handled, with non-cytotoxic environments suitable for general pharmaceutical or electronic production that requires less rigorous containment.
Table of Comparison
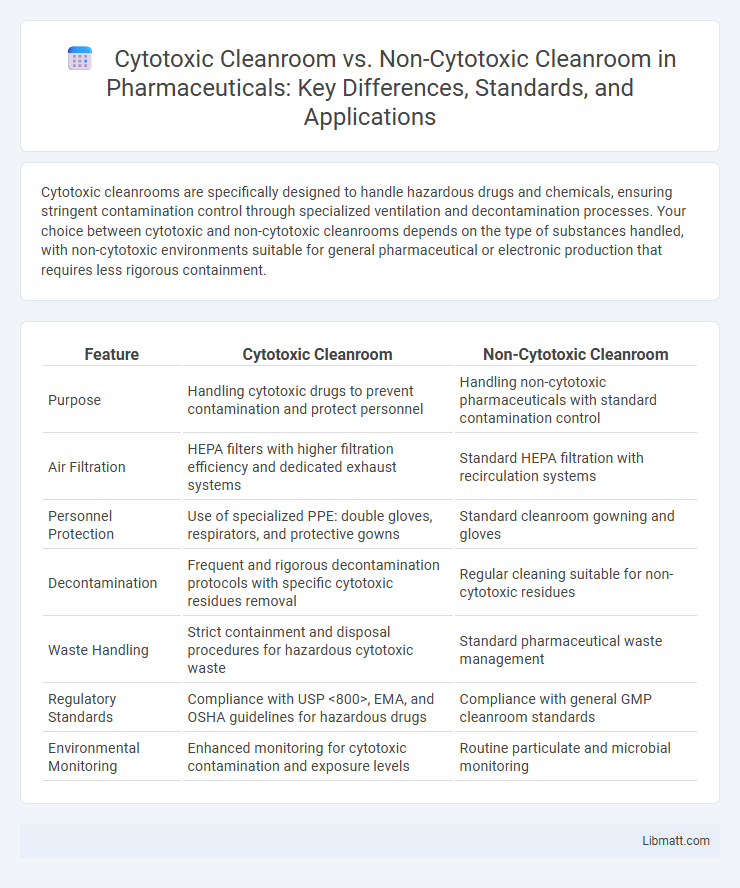
| Feature | Cytotoxic Cleanroom | Non-Cytotoxic Cleanroom |
|---|---|---|
| Purpose | Handling cytotoxic drugs to prevent contamination and protect personnel | Handling non-cytotoxic pharmaceuticals with standard contamination control |
| Air Filtration | HEPA filters with higher filtration efficiency and dedicated exhaust systems | Standard HEPA filtration with recirculation systems |
| Personnel Protection | Use of specialized PPE: double gloves, respirators, and protective gowns | Standard cleanroom gowning and gloves |
| Decontamination | Frequent and rigorous decontamination protocols with specific cytotoxic residues removal | Regular cleaning suitable for non-cytotoxic residues |
| Waste Handling | Strict containment and disposal procedures for hazardous cytotoxic waste | Standard pharmaceutical waste management |
| Regulatory Standards | Compliance with USP <800>, EMA, and OSHA guidelines for hazardous drugs | Compliance with general GMP cleanroom standards |
| Environmental Monitoring | Enhanced monitoring for cytotoxic contamination and exposure levels | Routine particulate and microbial monitoring |
Introduction to Cleanrooms in Pharmaceutical Manufacturing
In pharmaceutical manufacturing, cleanrooms are critical environments designed to control contamination and ensure product safety. Cytotoxic cleanrooms specifically handle hazardous drugs that require strict containment to protect both the product and personnel from toxic exposure. Non-cytotoxic cleanrooms manage less hazardous materials, focusing primarily on maintaining sterility and particulate control for general pharmaceutical production.
Defining Cytotoxic and Non-cytotoxic Cleanrooms
Cytotoxic cleanrooms are specialized controlled environments designed to handle hazardous substances such as chemotherapy drugs, minimizing contamination risks to protect both products and personnel. Non-cytotoxic cleanrooms focus on maintaining sterile conditions for general pharmaceutical or biotech manufacturing without the stringent containment requirements needed for cytotoxic agents. Understanding the distinction helps you select the appropriate cleanroom type based on the specific safety and contamination control needs of your operations.
Key Differences Between Cytotoxic and Non-cytotoxic Cleanrooms
Cytotoxic cleanrooms are specifically designed to handle hazardous drugs and materials that pose health risks, featuring specialized ventilation, HEPA filtration, and strict contamination control to protect workers and ensure product safety. Non-cytotoxic cleanrooms manage less hazardous substances, maintaining standard cleanliness levels without the enhanced protective measures needed for cytotoxic environments. Your choice between these cleanroom types depends on the nature of substances handled, regulatory compliance, and required safety protocols.
Regulatory Standards for Cytotoxic Cleanrooms
Cytotoxic cleanrooms must adhere to stringent regulatory standards such as ISO 14644 and USP <800> to ensure the safe handling of hazardous drugs and containment of toxic particles. These standards mandate specialized ventilation, rigorous contamination control, and personnel protective equipment to minimize exposure risks in your facility. Non-cytotoxic cleanrooms follow less restrictive guidelines, focusing primarily on general sterilization and particulate control without the added requirements for toxic substance management.
Safety Protocols in Cytotoxic Cleanroom Environments
Safety protocols in cytotoxic cleanroom environments rigorously enforce the use of specialized personal protective equipment (PPE) such as double gloves, respirators, and full-body suits to prevent exposure to hazardous antineoplastic drugs. Engineering controls including biological safety cabinets (BSCs) with HEPA filtration and negative pressure differentials maintain contamination containment and protect both personnel and product integrity. Comprehensive training programs and strict waste disposal procedures minimize contamination risks and ensure compliance with OSHA and USP <800> standards specific to cytotoxic agents.
Material Handling Procedures: Cytotoxic vs Non-cytotoxic
Material handling procedures in cytotoxic cleanrooms demand stringent controls to prevent exposure to hazardous agents, including the use of closed-system transfer devices, specialized personal protective equipment (PPE), and rigorous decontamination protocols. Non-cytotoxic cleanrooms employ standard material handling practices with less restrictive PPE and basic contamination control, reflecting lower health risks. The differentiation in handling protocols impacts workflow design, safety measures, and contamination risk mitigation strategies in pharmaceutical manufacturing environments.
Facility Design Considerations for Cytotoxic Cleanrooms
Facility design considerations for cytotoxic cleanrooms prioritize containment and safety to prevent exposure to hazardous drugs, requiring specialized ventilation systems with negative pressure zones and HEPA filtration. Materials used must resist chemical contamination, and personnel workflows are carefully planned to minimize cross-contamination and ensure compliant handling protocols. Your cleanroom must integrate robust waste management systems and comply with stringent regulatory standards such as USP <800> to protect workers and maintain product integrity.
Personnel Training Requirements
Personnel training in cytotoxic cleanrooms demands rigorous instruction on handling hazardous drugs, contamination control, and use of specialized personal protective equipment (PPE) to prevent exposure and ensure safety. Non-cytotoxic cleanrooms require training focused on standard contamination prevention, aseptic techniques, and general cleanroom protocols without the enhanced precautions necessary for cytotoxic agents. Proper training compliance reduces risks associated with cytotoxic drug handling and maintains regulatory standards such as USP <800> and OSHA guidelines.
Environmental Monitoring and Contamination Control
Cytotoxic cleanrooms require stringent environmental monitoring with specialized particle counters and surface sampling to detect hazardous drug residues, ensuring the highest contamination control standards. Non-cytotoxic cleanrooms implement standard microbial and particulate monitoring methods but with less rigorous control thresholds, focusing primarily on preventing general contamination. Your monitoring protocols must align with the product risk profile to mitigate cross-contamination and maintain compliance with regulatory guidelines.
Choosing the Right Cleanroom Type for your Application
Cytotoxic cleanrooms are specifically designed to handle hazardous pharmaceutical compounds, providing stringent containment and air purity standards essential for protecting workers and preventing contamination in drug manufacturing involving cytotoxic agents. Non-cytotoxic cleanrooms accommodate less hazardous processes, maintaining controlled environments primarily focused on particulate and microbial control rather than chemical toxicity. Choosing the right cleanroom type for your application depends on the nature of the substances handled, your regulatory compliance requirements, and the level of protection needed for personnel and products.
Cytotoxic Cleanroom vs Non-cytotoxic Cleanroom Infographic
 libmatt.com
libmatt.com