Emulsions are mixtures of two immiscible liquids where one is dispersed as droplets in the other, often stabilized by emulsifiers, while suspensions consist of solid particles dispersed in a liquid that may settle over time. Understanding the difference helps you choose the right formulation for applications in pharmaceuticals, cosmetics, or food products.
Table of Comparison
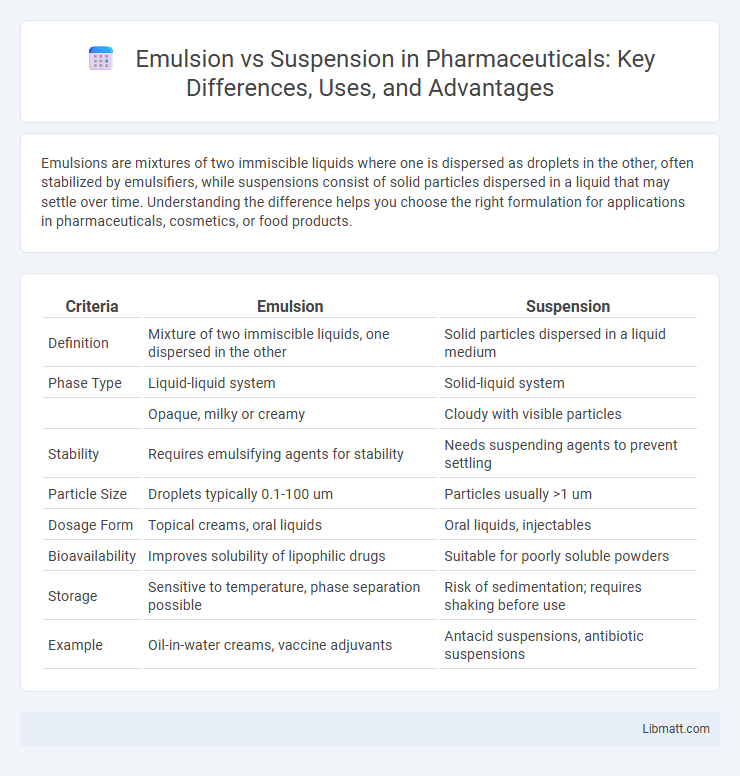
| Criteria | Emulsion | Suspension |
|---|---|---|
| Definition | Mixture of two immiscible liquids, one dispersed in the other | Solid particles dispersed in a liquid medium |
| Phase Type | Liquid-liquid system | Solid-liquid system |
| Opaque, milky or creamy | Cloudy with visible particles | |
| Stability | Requires emulsifying agents for stability | Needs suspending agents to prevent settling |
| Particle Size | Droplets typically 0.1-100 um | Particles usually >1 um |
| Dosage Form | Topical creams, oral liquids | Oral liquids, injectables |
| Bioavailability | Improves solubility of lipophilic drugs | Suitable for poorly soluble powders |
| Storage | Sensitive to temperature, phase separation possible | Risk of sedimentation; requires shaking before use |
| Example | Oil-in-water creams, vaccine adjuvants | Antacid suspensions, antibiotic suspensions |
Introduction to Emulsions and Suspensions
Emulsions are heterogeneous mixtures consisting of two immiscible liquids where one liquid is dispersed as small droplets within the other, commonly stabilized by emulsifiers to prevent separation. Suspensions are heterogeneous mixtures containing solid particles dispersed in a liquid medium, where particles are typically larger and tend to settle over time without stabilizers. Both emulsions and suspensions display phase separation but differ in the physical state of their dispersed components and their stabilization mechanisms.
Definition and Key Differences
An emulsion is a mixture of two immiscible liquids where one liquid is dispersed in the form of tiny droplets within the other, commonly stabilized by emulsifying agents, while a suspension consists of solid particles dispersed throughout a liquid medium that settle over time. Key differences include particle size, with emulsions having smaller droplets typically less than 100 nanometers, and suspensions containing larger solid particles that can be seen with the naked eye. Emulsions are generally cloudy or opaque but stable, whereas suspensions tend to be heterogeneous and require shaking to redistribute particles.
Composition and Structure
Emulsions consist of two immiscible liquids, such as oil and water, where one liquid is dispersed as tiny droplets within the other, forming a stable colloidal system often stabilized by emulsifiers. Suspensions are heterogeneous mixtures containing solid particles dispersed within a liquid medium, with suspended particles large enough to settle over time due to gravity. The structural difference lies in emulsions having liquid-liquid interfaces stabilized by surfactants, while suspensions involve solid-liquid interactions without molecular-level dispersion.
Mechanisms of Formation
Emulsion formation involves the dispersion of two immiscible liquids, typically oil and water, stabilized by emulsifying agents that reduce interfacial tension and prevent phase separation. Suspension formation occurs when solid particles are dispersed in a liquid medium, relying on agitation and stabilizers to maintain uniform particle distribution and prevent sedimentation. Both mechanisms depend on factors like particle size, surface properties, and the presence of surfactants or stabilizers to ensure stability and homogeneity.
Stability and Separation
Emulsions exhibit greater stability than suspensions because their dispersed droplets are often stabilized by emulsifying agents that prevent coalescence, reducing the rate of separation. Suspensions tend to separate quickly due to larger particle sizes that settle under gravity, causing sedimentation and requiring frequent shaking or stirring to maintain uniformity. Understanding the differences in stability and separation between emulsions and suspensions helps you optimize formulation strategies for your desired application.
Common Examples and Applications
Emulsions commonly include mayonnaise and milk, widely used in food processing for texture and flavor enhancement. Suspensions such as muddy water and pharmaceutical suspensions are crucial in industrial applications and medicine for delivering active ingredients effectively. Both emulsions and suspensions play vital roles in cosmetics, paint formulations, and chemical manufacturing due to their distinct particle dispersion characteristics.
Methods of Preparation
Emulsions are typically prepared using high-shear mixing or ultrasonic emulsification, which disperses one liquid phase into another immiscible liquid, often stabilized by surfactants or emulsifying agents. Suspensions are created by mechanically dispersing insoluble solid particles uniformly throughout a liquid medium using stirring or homogenization, sometimes aided by wetting agents to prevent particle aggregation. Both methods require careful control of particle size and distribution to ensure stability and desired properties in pharmaceutical or food applications.
Industrial and Pharmaceutical Uses
Emulsions and suspensions serve distinct purposes in industrial and pharmaceutical applications, with emulsions commonly used in cosmetics, food processing, and drug delivery systems due to their stable mixtures of immiscible liquids. Suspensions are preferred for formulations requiring solid particles dispersed in liquids, such as antibiotics and antacids, allowing controlled release and ease of application. Your choice between the two depends on the desired stability, texture, and release characteristics critical in manufacturing and therapeutic effectiveness.
Advantages and Limitations
Emulsions offer enhanced stability and uniform texture, making them ideal for delivering water-insoluble substances in pharmaceuticals and cosmetics, but they require emulsifying agents and can be sensitive to temperature changes. Suspensions allow for easier formulation of insoluble solid particles with adjustable particle sizes and straightforward manufacturing, though they may settle over time, requiring shaking before use and often having limited shelf life. Your choice depends on the specific application needs, balancing the emulsion's smooth consistency against the suspension's simplicity and ease of preparation.
Conclusion and Selection Criteria
Choosing between an emulsion and a suspension depends primarily on the desired stability and particle size distribution of the mixture. Emulsions, consisting of two immiscible liquids with one dispersed as droplets within the other, offer better uniformity and stability for applications in pharmaceuticals and cosmetics, whereas suspensions, containing solid particles dispersed in a liquid, are preferred for formulations needing easy sedimentation and redispersibility. Selection criteria include the nature of the active ingredients, intended shelf life, administration route, and required homogeneity, with emulsions favored for consistent texture and suspensions for more straightforward preparation and cost-effectiveness.
Emulsion vs suspension Infographic
 libmatt.com
libmatt.com