Change control focuses on managing and approving planned changes to processes or products to ensure quality and compliance, while deviation control handles unplanned departures from established standards or procedures, investigating causes and implementing corrective actions. Your organization benefits from applying change control to systematically introduce improvements and deviation control to promptly address and rectify unexpected issues.
Table of Comparison
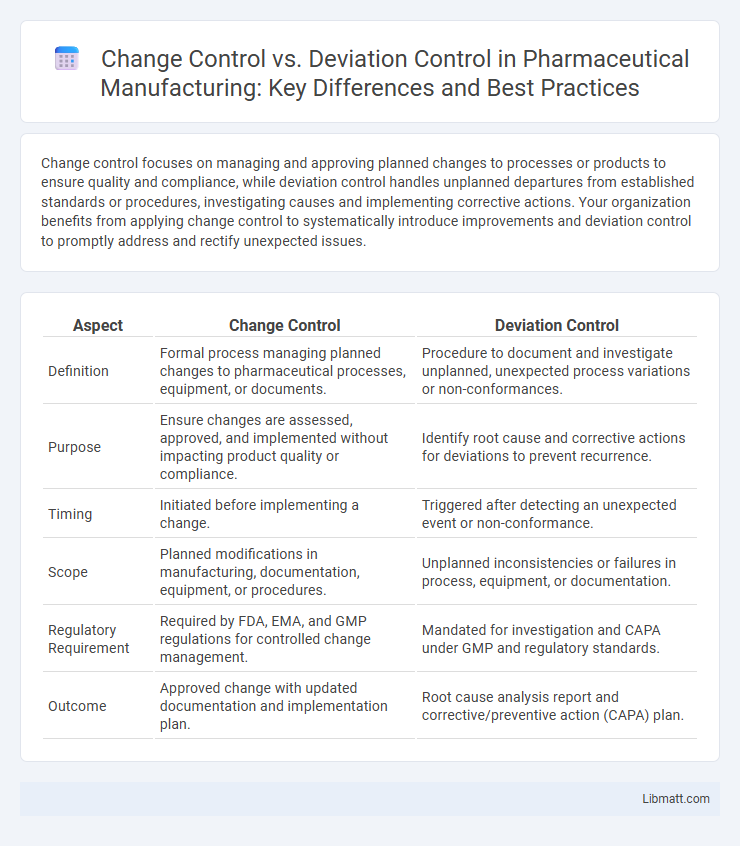
| Aspect | Change Control | Deviation Control |
|---|---|---|
| Definition | Formal process managing planned changes to pharmaceutical processes, equipment, or documents. | Procedure to document and investigate unplanned, unexpected process variations or non-conformances. |
| Purpose | Ensure changes are assessed, approved, and implemented without impacting product quality or compliance. | Identify root cause and corrective actions for deviations to prevent recurrence. |
| Timing | Initiated before implementing a change. | Triggered after detecting an unexpected event or non-conformance. |
| Scope | Planned modifications in manufacturing, documentation, equipment, or procedures. | Unplanned inconsistencies or failures in process, equipment, or documentation. |
| Regulatory Requirement | Required by FDA, EMA, and GMP regulations for controlled change management. | Mandated for investigation and CAPA under GMP and regulatory standards. |
| Outcome | Approved change with updated documentation and implementation plan. | Root cause analysis report and corrective/preventive action (CAPA) plan. |
Introduction to Change Control and Deviation Control
Change control is a structured process ensuring all alterations to processes or products are reviewed, approved, and documented to maintain consistency and compliance. Deviation control addresses unexpected events or non-conformances by identifying, investigating, and managing exceptions to prevent impact on quality or safety. Your company benefits by implementing both controls to maintain regulatory standards and ensure product integrity throughout production.
Defining Change Control in Quality Management
Change control in quality management systematically manages alterations to processes, documents, or products to ensure compliance with regulatory standards and maintain product integrity. It involves evaluating proposed changes through formal review processes, including risk assessment, approval, implementation, and documentation to prevent unintended consequences. Deviation control, in contrast, addresses unplanned departures from established procedures, focusing on identifying root causes and corrective actions to restore conformity.
Understanding Deviation Control Processes
Deviation control processes focus on identifying, documenting, and managing departures from approved procedures or specifications to maintain product quality and compliance. These processes enable your team to investigate root causes, assess impact, and implement corrective actions to prevent recurrence. Effective deviation control ensures regulatory adherence and continuous improvement within quality management systems.
Key Differences between Change Control and Deviation Control
Change control manages planned modifications to processes, ensuring systematic evaluation and approval before implementation, while deviation control addresses unplanned, unexpected departures from established procedures that require immediate correction. Change control emphasizes risk assessment and documentation to prevent issues, whereas deviation control focuses on root cause analysis and corrective actions to restore compliance. Your organization's quality management system benefits from understanding these distinctions to maintain regulatory adherence and product integrity.
Importance of Robust Change Control Procedures
Robust change control procedures are critical for maintaining quality and compliance in regulated industries by ensuring all modifications are systematically evaluated, documented, and approved before implementation. Effective change control minimizes risks associated with unintended consequences, protecting product integrity and patient safety. Your organization benefits from enhanced traceability and accountability, reducing errors and regulatory scrutiny compared to less stringent deviation control processes.
Managing and Documenting Deviations Effectively
Managing and documenting deviations effectively requires a clear distinction between change control and deviation control processes. Change control focuses on planned modifications to processes, ensuring systematic evaluation and approval before implementation, while deviation control addresses unplanned departures from established procedures, emphasizing thorough documentation, root cause analysis, and corrective actions. Effective deviation management involves capturing detailed records, assessing impact on product quality and compliance, and implementing preventive measures to minimize recurrence.
Regulatory Requirements for Change and Deviation Controls
Regulatory requirements for change control mandate thorough documentation, risk assessment, and approval processes to ensure modifications do not compromise product quality or compliance with standards such as FDA 21 CFR Part 820 and EMA guidelines. Deviation control regulations require immediate identification, investigation, and corrective actions for any departures from approved procedures or specifications to maintain compliance and prevent product recalls. Both controls must be integrated into quality management systems with traceable records to satisfy audits and inspections by regulatory bodies.
Common Challenges in Change and Deviation Management
Change and deviation control processes often face common challenges such as inadequate documentation, lack of timely communication, and inconsistent risk assessment, which can lead to compliance issues and operational delays. Ensuring your team adheres to standardized procedures and maintains thorough records is critical for effective management and regulatory approval. Misalignment between change and deviation controls can also complicate root cause analysis and corrective action implementation.
Best Practices for Integrating Change and Deviation Controls
Integrating change control and deviation control effectively requires establishing clear protocols that differentiate planned modifications from unplanned variances, ensuring regulatory compliance and quality assurance. Implement robust documentation and approval workflows that allow your team to track deviations promptly and evaluate their impact before implementing changes. Aligning these controls within a unified quality management system enhances traceability and minimizes risks associated with product or process alterations.
Conclusion: Aligning Change and Deviation Controls for Compliance
Aligning change control and deviation control processes ensures regulatory compliance by systematically addressing planned modifications and unplanned anomalies within quality systems. Integrating both controls enhances traceability, risk management, and documentation accuracy, crucial for meeting FDA and ISO standards. A harmonized approach mitigates operational disruptions and supports continuous improvement in regulated environments.
Change control vs Deviation control Infographic
 libmatt.com
libmatt.com