Ion exchange chromatography separates molecules based on their charge by using a charged resin that attracts oppositely charged ions, making it ideal for purifying proteins and nucleic acids with different isoelectric points. Affinity chromatography exploits specific binding interactions between a target molecule and a ligand attached to the resin, offering high selectivity for purifying proteins, enzymes, or antibodies with particular affinity tags or binding sites.
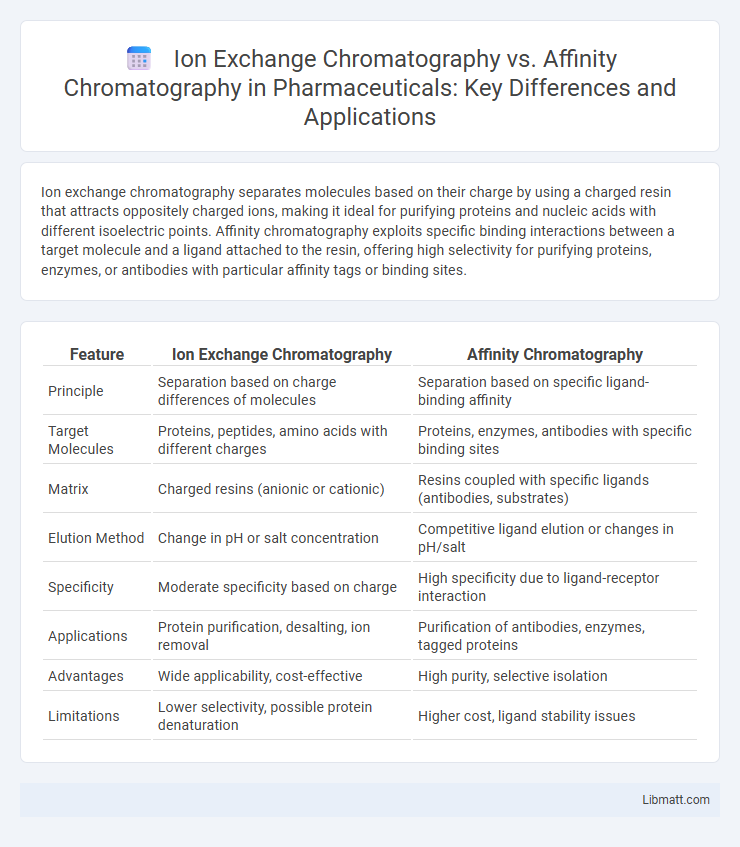
Table of Comparison
| Feature | Ion Exchange Chromatography | Affinity Chromatography |
|---|---|---|
| Principle | Separation based on charge differences of molecules | Separation based on specific ligand-binding affinity |
| Target Molecules | Proteins, peptides, amino acids with different charges | Proteins, enzymes, antibodies with specific binding sites |
| Matrix | Charged resins (anionic or cationic) | Resins coupled with specific ligands (antibodies, substrates) |
| Elution Method | Change in pH or salt concentration | Competitive ligand elution or changes in pH/salt |
| Specificity | Moderate specificity based on charge | High specificity due to ligand-receptor interaction |
| Applications | Protein purification, desalting, ion removal | Purification of antibodies, enzymes, tagged proteins |
| Advantages | Wide applicability, cost-effective | High purity, selective isolation |
| Limitations | Lower selectivity, possible protein denaturation | Higher cost, ligand stability issues |
Introduction to Chromatography Techniques
Ion exchange chromatography separates molecules based on their charge differences by utilizing charged resins to attract oppositely charged ions, making it ideal for purifying proteins with varying isoelectric points. Affinity chromatography exploits specific biological interactions between a target molecule and an immobilized ligand, allowing highly selective purification based on binding affinity. Your choice between these techniques depends on the nature of the sample and the required specificity for efficient separation and analysis.
Principles of Ion Exchange Chromatography
Ion exchange chromatography separates molecules based on their net surface charge by exploiting the reversible binding between charged ions on the stationary phase and oppositely charged analytes. This method uses cation or anion exchangers to attract and retain target molecules, which are subsequently eluted by changing pH or ionic strength. Your choice between ion exchange and affinity chromatography depends on the specific charge properties and binding affinities of the biomolecules you need to purify.
Fundamentals of Affinity Chromatography
Affinity chromatography relies on the highly specific interaction between a target molecule and a ligand attached to a stationary phase, enabling selective purification based on biological affinity. This method exploits reversible binding, often involving antibodies, enzymes, or receptors, to capture and subsequently elute the target protein under mild conditions. Compared to ion exchange chromatography, which separates molecules based on charge differences, affinity chromatography provides greater specificity for isolating biomolecules such as proteins, nucleic acids, or other ligands.
Comparison of Mechanisms: Ion Exchange vs Affinity
Ion exchange chromatography separates molecules based on their net surface charge by utilizing charged resin beads that attract oppositely charged analytes, whereas affinity chromatography exploits specific binding interactions between a target molecule and an immobilized ligand on the stationary phase. In ion exchange, separation depends on electrostatic interactions affected by pH and ionic strength, while affinity chromatography relies on high specificity and biological recognition, such as antigen-antibody or enzyme-substrate interactions. Your choice between these methods depends on whether you need broad separation based on charge differences or precise purification targeting a specific biomolecule.
Applications in Protein Purification
Ion exchange chromatography excels in purifying proteins based on charge differences, making it ideal for separating isoforms and removing contaminants in large-scale protein production. Affinity chromatography offers high specificity by exploiting protein-ligand interactions, frequently used for purifying enzymes, antibodies, and recombinant proteins with tagged epitopes. Your choice depends on purity requirements and protein properties, with ion exchange suited for initial bulk purification and affinity chromatography for final, high-purity isolation.
Key Factors Affecting Separation Efficiency
Separation efficiency in ion exchange chromatography is primarily influenced by factors such as pH, ionic strength, and the nature of the resin, which modulate the electrostatic interactions between charged analytes and the stationary phase. In affinity chromatography, the specificity and strength of the ligand-analyte interaction, as well as the binding capacity and elution conditions, play crucial roles in determining separation efficiency. Optimizing these parameters in your purification process ensures maximum yield and purity for target biomolecules.
Advantages and Limitations of Ion Exchange Chromatography
Ion exchange chromatography offers high resolution and capacity for separating proteins based on charge differences, making it ideal for purifying charged biomolecules. It is cost-effective and scalable for industrial use but has limitations such as sensitivity to pH and ionic strength changes, which can affect binding specificity and protein stability. Additionally, ion exchange cannot selectively isolate target proteins without prior knowledge of their charge properties, unlike affinity chromatography.
Strengths and Weaknesses of Affinity Chromatography
Affinity chromatography offers exceptional specificity and high purity by selectively binding target molecules through biological interactions, making it ideal for protein purification and enzyme isolation. However, its limitations include high cost due to expensive ligands and potential ligand leakage, as well as limited versatility since it requires prior knowledge of the target molecule's binding properties. Compared to ion exchange chromatography, which is more cost-effective and broadly applicable, affinity chromatography excels in selectivity but may face scalability and reusability challenges.
Choosing the Right Technique for Biomolecule Isolation
Ion exchange chromatography separates biomolecules based on their net charge by exploiting electrostatic interactions with charged resin, making it ideal for proteins or nucleic acids with distinct isoelectric points. Affinity chromatography offers high specificity by using ligands that selectively bind target biomolecules, perfect for purifying enzymes, antibodies, or receptors with known binding partners. Selecting the right technique depends on the target molecule's properties, desired purity, and binding affinity to ensure efficient isolation and optimal yield.
Future Trends in Chromatographic Separation
Future trends in chromatographic separation emphasize hybrid techniques that combine ion exchange chromatography and affinity chromatography to enhance selectivity and capacity. Advances in smart materials and molecular recognition elements are driving the development of more robust and reusable affinity ligands. Integration with automated, high-throughput systems and real-time monitoring technologies is expected to revolutionize precision in protein purification and bioseparation processes.
Ion exchange chromatography vs affinity chromatography Infographic
 libmatt.com
libmatt.com