Oral solids offer the convenience of self-administration and controlled release, making them ideal for long-term therapy, while parenterals provide rapid drug absorption and precise dosing crucial for emergency or inpatient care. Your choice between oral solids and parenterals depends on factors such as onset of action, bioavailability, and patient compliance.
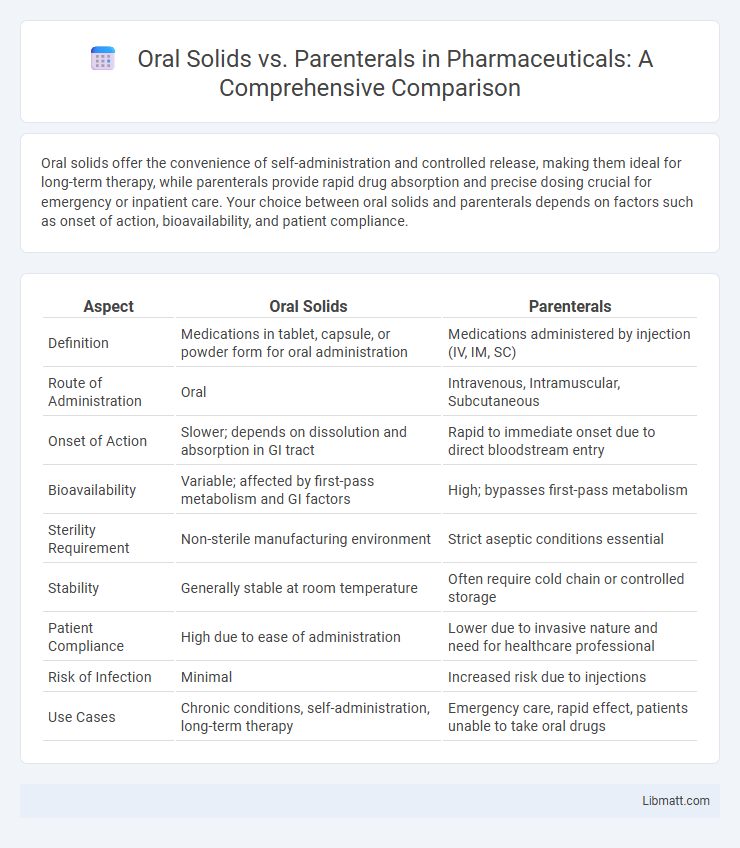
Table of Comparison
| Aspect | Oral Solids | Parenterals |
|---|---|---|
| Definition | Medications in tablet, capsule, or powder form for oral administration | Medications administered by injection (IV, IM, SC) |
| Route of Administration | Oral | Intravenous, Intramuscular, Subcutaneous |
| Onset of Action | Slower; depends on dissolution and absorption in GI tract | Rapid to immediate onset due to direct bloodstream entry |
| Bioavailability | Variable; affected by first-pass metabolism and GI factors | High; bypasses first-pass metabolism |
| Sterility Requirement | Non-sterile manufacturing environment | Strict aseptic conditions essential |
| Stability | Generally stable at room temperature | Often require cold chain or controlled storage |
| Patient Compliance | High due to ease of administration | Lower due to invasive nature and need for healthcare professional |
| Risk of Infection | Minimal | Increased risk due to injections |
| Use Cases | Chronic conditions, self-administration, long-term therapy | Emergency care, rapid effect, patients unable to take oral drugs |
Overview of Oral Solids and Parenterals
Oral solids, including tablets and capsules, offer convenience, precise dosing, and enhanced patient compliance, making them the most common dosage forms in pharmaceutical therapy. Parenterals, encompassing injections and infusions, provide rapid drug delivery and 100% bioavailability by bypassing the gastrointestinal tract, essential for emergency treatments and drugs with poor oral absorption. The choice between oral solids and parenterals depends on factors like drug stability, onset of action, and patient-specific needs for effective therapeutic outcomes.
Definition and Forms of Oral Solid Dosage
Oral solid dosage forms include tablets, capsules, powders, and granules designed for administration via the mouth, providing convenience and precise dosing. These forms enhance stability and shelf life compared to liquid parenteral formulations, making them ideal for chronic conditions. Tablets often incorporate binders and disintegrants to ensure proper dissolution and bioavailability, while capsules can contain powders or pellets for controlled release.
Parenteral Administration: Types and Techniques
Parenteral administration involves delivering medications directly into the body through injections, bypassing the gastrointestinal tract for rapid systemic effects. Common types include intravenous, intramuscular, subcutaneous, and intradermal injections, each varying in absorption speed and target tissue. Understanding these techniques ensures your treatment is effective, especially when oral solids are unsuitable due to absorption issues or emergencies.
Bioavailability: Oral Solids vs Parenterals
Parenteral drugs typically exhibit higher bioavailability compared to oral solids due to direct entry into systemic circulation, bypassing first-pass metabolism. Oral solids undergo variable absorption influenced by gastrointestinal pH, enzyme activity, and intestinal transit time, often resulting in reduced and inconsistent bioavailability. The bioavailability of oral solids is further limited by solubility and permeability factors, whereas parenterals provide precise dosing with rapid onset of action.
Patient Compliance and Convenience
Oral solids, such as tablets and capsules, significantly enhance patient compliance due to ease of administration, portability, and convenience for self-dosing without the need for professional assistance. Parenteral formulations, including injections and infusions, often require healthcare provider involvement, sterile conditions, and can cause discomfort or pain, which may reduce patient adherence. The simplicity of oral solids supports chronic treatment regimens by facilitating consistent intake, whereas parenterals are typically reserved for acute conditions or when rapid bioavailability is essential.
Stability and Shelf Life Considerations
Oral solids generally offer greater stability and longer shelf life compared to parenterals due to their solid-state formulation, which reduces degradation caused by moisture and temperature fluctuations. Parenterals require stringent storage conditions and have shorter shelf lives because their liquid form is more susceptible to microbial contamination and chemical instability. When managing your medication inventory, prioritizing oral solids can simplify storage while ensuring extended product efficacy.
Safety and Sterility Concerns
Oral solids, such as tablets and capsules, generally pose fewer sterility concerns due to their exposure to the gastrointestinal tract, which has natural defense mechanisms, while parenterals require strict aseptic processing to prevent microbial contamination since they bypass these barriers and enter directly into systemic circulation. Safety risks with oral solids primarily involve dose uniformity and stability, whereas parenterals demand rigorous sterility testing, endotoxin control, and particulate matter exclusion to avoid severe adverse reactions like infections or embolism. Regulatory guidelines for parenteral products emphasize stringent Good Manufacturing Practices (GMP) to ensure sterility assurance levels not typically mandated for oral solid dosage forms.
Cost and Manufacturing Implications
Oral solids typically incur lower manufacturing costs due to simpler production processes and easier packaging, requiring less specialized equipment compared to parenterals. Parenteral formulations demand stringent sterile environments, higher quality control standards, and complex aseptic processing, increasing production expenses significantly. The cost of raw materials, regulatory compliance, and specialized storage conditions further elevate the overall manufacturing investment for parenteral drugs relative to oral solids.
Clinical Applications and Indications
Oral solids are primarily used for chronic conditions requiring sustained drug release, such as hypertension, diabetes, and chronic pain management, offering ease of administration and patient compliance. Parenterals are favored in acute care, emergency situations, and when rapid drug action or precise dosing is critical, including treatments for infections, anesthesia, and chemotherapy. Your choice between oral solids and parenterals depends on factors like onset of action, bioavailability, and the clinical condition requiring targeted therapy.
Future Trends in Oral Solids and Parenterals
Future trends in oral solids emphasize advanced drug delivery systems such as controlled-release formulations and personalized medicine driven by 3D printing technology. Parenteral drug development is progressing toward minimally invasive injectable devices, including microneedle patches and long-acting injectables for improved patient compliance. Both oral solids and parenterals are incorporating nanotechnology and targeted delivery to enhance bioavailability and therapeutic efficacy.
Oral solids vs Parenterals Infographic
 libmatt.com
libmatt.com