Shelf-life indicates the period during which a product maintains its intended quality and safety under proper storage conditions, while the expiry date specifically marks the last day the product is guaranteed to be safe and effective to use. Understanding the distinction helps you make informed decisions about product usability and prevent potential health risks.
Table of Comparison
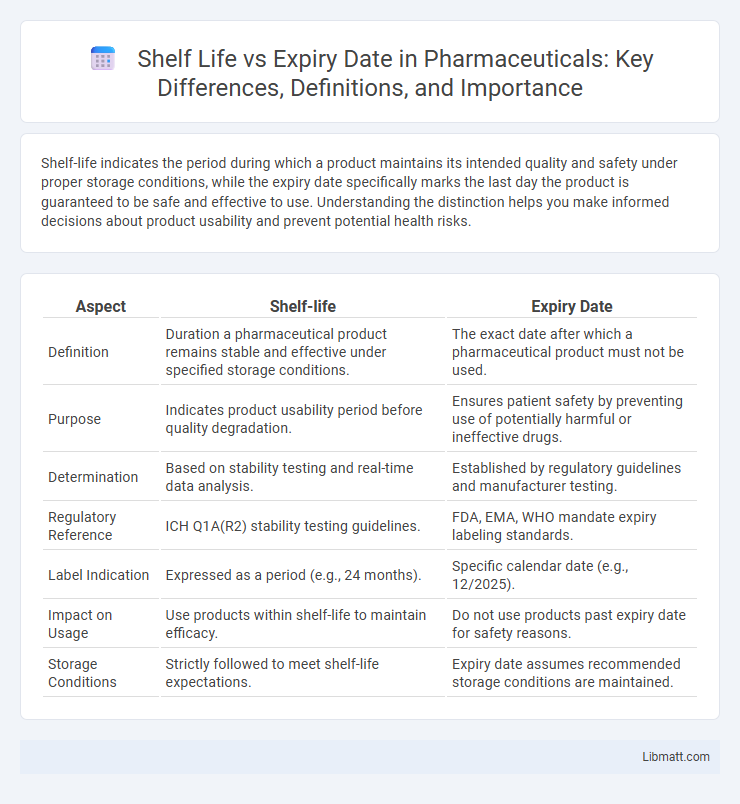
| Aspect | Shelf-life | Expiry Date |
|---|---|---|
| Definition | Duration a pharmaceutical product remains stable and effective under specified storage conditions. | The exact date after which a pharmaceutical product must not be used. |
| Purpose | Indicates product usability period before quality degradation. | Ensures patient safety by preventing use of potentially harmful or ineffective drugs. |
| Determination | Based on stability testing and real-time data analysis. | Established by regulatory guidelines and manufacturer testing. |
| Regulatory Reference | ICH Q1A(R2) stability testing guidelines. | FDA, EMA, WHO mandate expiry labeling standards. |
| Label Indication | Expressed as a period (e.g., 24 months). | Specific calendar date (e.g., 12/2025). |
| Impact on Usage | Use products within shelf-life to maintain efficacy. | Do not use products past expiry date for safety reasons. |
| Storage Conditions | Strictly followed to meet shelf-life expectations. | Expiry date assumes recommended storage conditions are maintained. |
Understanding Shelf-Life and Expiry Date
Shelf-life refers to the period during which a product maintains its intended quality and safety under specified storage conditions, while the expiry date indicates the last date a product is guaranteed to be safe and effective. Understanding these differences is critical for proper inventory management, consumer safety, and regulatory compliance in industries such as pharmaceuticals, food, and cosmetics. Products can often be used safely and effectively within the shelf-life but should never be consumed or applied past their expiry date to avoid health risks.
Key Differences Between Shelf-Life and Expiry Date
Shelf-life refers to the period during which a product maintains its desired quality and safety under specified storage conditions, while the expiry date marks the point after which the product is no longer guaranteed to be safe or effective for use. Shelf-life focuses on stability and usability span, whereas the expiry date is a strict deadline indicating potential health risks if the product is consumed. Understanding these key differences helps you make informed decisions about product storage and usage to ensure safety and effectiveness.
Why Shelf-Life Matters in Product Safety
Shelf-life is a critical factor in product safety as it indicates the period during which a product maintains its intended quality and effectiveness under specified storage conditions. Expiry dates mark the point beyond which product safety and potency cannot be guaranteed, directly impacting consumer health risk. Understanding shelf-life helps manufacturers establish safe usage timelines, preventing the distribution of contaminated or degraded goods.
How Expiry Dates Are Determined
Expiry dates are determined based on rigorous stability testing that assesses how long a product maintains its safety, potency, and quality under specific storage conditions. Regulatory agencies require manufacturers to conduct these tests using standardized protocols, including accelerated and real-time studies, to predict the product's degradation timeline. This data ensures expiry dates accurately reflect the period during which the product remains effective and safe for consumer use.
Factors Affecting Shelf-Life
Shelf-life is influenced by factors including temperature, humidity, light exposure, and packaging materials, all of which impact product stability and safety. Chemical composition, microbial activity, and storage conditions play critical roles in determining how long a product retains its quality before reaching its expiry date. Proper control of these variables extends shelf-life, ensuring optimal performance and consumer safety.
Interpreting Shelf-Life Labels Correctly
Shelf-life labels indicate the period a product maintains optimal quality under proper storage, while expiry dates mark the point after which safety and efficacy may be compromised. Correct interpretation involves understanding that shelf-life is about maintaining freshness and usability, not necessarily safety, unlike expiry dates which signal a strict consumption deadline. Consumers should follow storage instructions closely and use products before the expiry date to ensure both quality and safety are preserved.
Myths and Facts About Expiry Dates
Expiry dates are often misunderstood as strict indicators of product safety, but many goods remain safe and effective beyond the printed date if stored properly. Shelf-life refers to the period a product maintains its intended quality, whereas expiry dates legally mark the end of guaranteed safety or efficacy. Myths such as all expired products being unsafe can lead to unnecessary waste, while facts show that certain items, especially dry or canned goods, can remain usable well past their expiry if packaging is intact.
Shelf-Life vs Expiry Date: Consumer Implications
Shelf-life defines the period during which a product maintains its intended quality and safety under specified storage conditions, while the expiry date indicates the point after which the product should not be used for safety reasons. Consumers using products beyond the expiry date risk exposure to degraded quality, reduced efficacy, or potential health hazards. Understanding the distinction helps consumers make informed decisions about product usage, minimizing health risks and ensuring optimal performance.
Proper Storage to Maximize Shelf-Life
Proper storage conditions, such as maintaining consistent temperature and humidity, are crucial to maximizing the shelf-life of perishable goods. Exposure to air, light, and moisture can accelerate spoilage, reducing the product's effectiveness before the expiry date. Understanding your item's specific storage requirements ensures its quality and safety are preserved for the duration of its shelf-life.
Regulatory Guidelines for Shelf-Life and Expiry Date
Regulatory guidelines for shelf-life and expiry date vary across industries but consistently require manufacturers to conduct stability testing to determine product safety and efficacy over time. Regulatory bodies such as the FDA, EMA, and WHO mandate clear labeling of expiry dates to ensure consumer protection and compliance with quality standards. Your products must meet these regulations by accurately documenting shelf-life data to avoid legal issues and maintain market trust.
Shelf-life vs Expiry date Infographic
 libmatt.com
libmatt.com