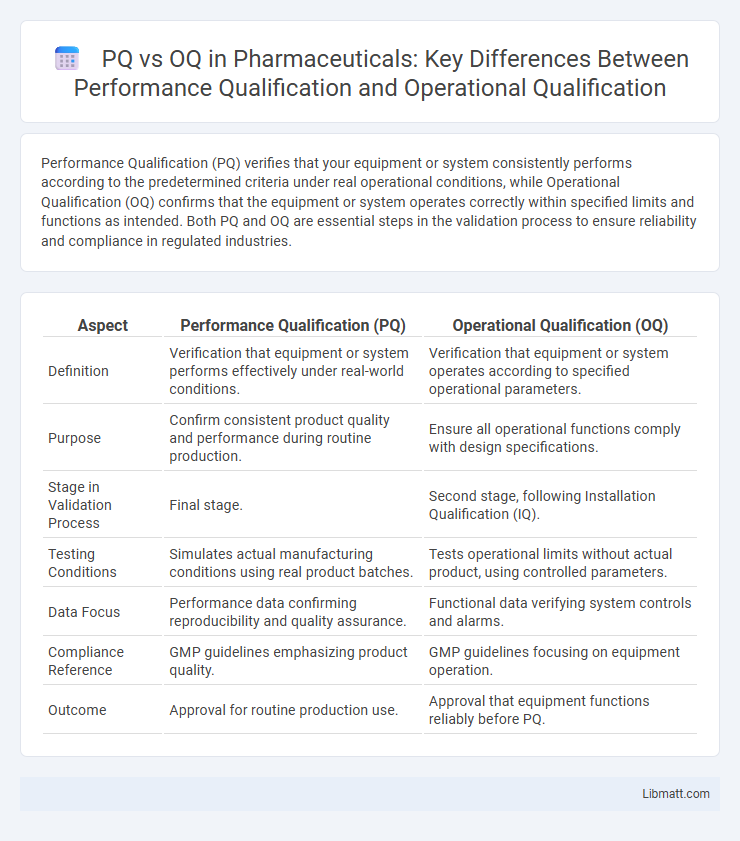
Performance Qualification (PQ) verifies that your equipment or system consistently performs according to the predetermined criteria under real operational conditions, while Operational Qualification (OQ) confirms that the equipment or system operates correctly within specified limits and functions as intended. Both PQ and OQ are essential steps in the validation process to ensure reliability and compliance in regulated industries.
Table of Comparison
| Aspect | Performance Qualification (PQ) | Operational Qualification (OQ) |
|---|---|---|
| Definition | Verification that equipment or system performs effectively under real-world conditions. | Verification that equipment or system operates according to specified operational parameters. |
| Purpose | Confirm consistent product quality and performance during routine production. | Ensure all operational functions comply with design specifications. |
| Stage in Validation Process | Final stage. | Second stage, following Installation Qualification (IQ). |
| Testing Conditions | Simulates actual manufacturing conditions using real product batches. | Tests operational limits without actual product, using controlled parameters. |
| Data Focus | Performance data confirming reproducibility and quality assurance. | Functional data verifying system controls and alarms. |
| Compliance Reference | GMP guidelines emphasizing product quality. | GMP guidelines focusing on equipment operation. |
| Outcome | Approval for routine production use. | Approval that equipment functions reliably before PQ. |
Introduction to Qualification in Regulated Industries
Qualification in regulated industries ensures equipment and systems meet predefined criteria for quality and compliance. PQ (Performance Qualification) verifies that systems consistently perform according to operational requirements under real-world conditions, while OQ (Operational Qualification) confirms that equipment operates correctly across all specified parameters. Your understanding of PQ vs OQ is crucial for maintaining validation integrity and meeting regulatory standards.
Defining Operational Qualification (OQ)
Operational Qualification (OQ) verifies that equipment and systems operate consistently according to specified parameters and within defined tolerances, ensuring they perform as intended under simulated operational conditions. It is a critical phase following Installation Qualification (IQ) and preceding Performance Qualification (PQ) in the validation process. Your successful OQ confirms that processes function correctly before progressing to evaluate overall system performance in real production scenarios.
Understanding Performance Qualification (PQ)
Performance Qualification (PQ) validates that equipment and systems consistently perform according to defined criteria under real-world conditions, ensuring product quality and process reliability. PQ follows Operational Qualification (OQ), which confirms that equipment operates within specified parameters during controlled testing. Together, PQ and OQ form essential phases in the validation process, critical for regulatory compliance and optimized manufacturing performance.
Key Differences Between PQ and OQ
Performance Qualification (PQ) verifies that a system or equipment consistently performs according to predefined criteria under real-world conditions, whereas Operational Qualification (OQ) ensures that the equipment operates within specified limits under controlled conditions. PQ focuses on validating actual process performance, including end-to-end operational functionality, while OQ tests the equipment's operational parameters and functions against design specifications. OQ is typically conducted before PQ, forming a foundational step in the overall validation process to guarantee readiness for performance testing.
Purpose of OQ in Equipment Validation
Operational Qualification (OQ) in equipment validation is designed to verify that the equipment operates according to predefined specifications under simulated or actual operating conditions. The primary purpose of OQ is to ensure that all operational parameters function correctly and consistently within established limits, confirming the reliability and accuracy of the equipment. This phase establishes documented evidence that the system performs as intended before proceeding to Performance Qualification (PQ), which focuses on confirming consistent performance in actual production conditions.
Role of PQ in Ensuring Process Performance
Performance Qualification (PQ) validates that equipment and systems consistently perform according to predetermined criteria under real-world conditions, ensuring the process meets its intended purpose. PQ assesses product quality and process reliability through actual production scenarios, while Operational Qualification (OQ) verifies equipment operation within specified parameters. Your confidence in process performance is strengthened by PQ's role in confirming sustained and reproducible outcomes during routine manufacturing.
Typical Steps in OQ Procedures
Operational Qualification (OQ) procedures typically include verifying that equipment and systems operate according to specified parameters under simulated operational conditions. Common steps involve calibrating instruments, running system diagnostics, and documenting performance against acceptance criteria. These procedures ensure consistent operation before progressing to Performance Qualification (PQ), which validates equipment under actual process conditions.
Essential Components of PQ Activities
Performance Qualification (PQ) activities focus on verifying that a system or equipment consistently performs according to predefined criteria under real-world conditions, emphasizing actual production parameters and environmental settings. Essential components of PQ include validating product quality attributes, confirming operational efficiency over extended runs, and documenting reproducibility of performance using process-specific acceptance criteria. PQ ensures the system's capability to deliver consistent results by assessing equipment functionality during normal operating cycles, integrating factors such as load variations, operator influence, and system responses.
Regulatory Requirements: OQ vs PQ
Operational Qualification (OQ) verifies that equipment operates within predetermined limits under simulated operating conditions, meeting regulatory requirements for performance consistency. Performance Qualification (PQ) demonstrates that systems consistently produce results meeting specifications during actual production, ensuring compliance with GMP and FDA guidelines. Your validation strategy must address both OQ and PQ stages to satisfy regulatory expectations and maintain product quality.
Best Practices for Seamless OQ and PQ Integration
Seamless integration of Performance Qualification (PQ) and Operational Qualification (OQ) requires aligning OQ protocols with the definitive performance criteria established during PQ to ensure coherent validation outcomes. Best practices emphasize meticulous documentation, synchronized scheduling, and cross-functional team collaboration to verify that equipment operates consistently under actual production conditions. You should implement real-time monitoring and robust data analysis during OQ to identify potential deviations early, facilitating a smoother transition into PQ and minimizing validation risks.
PQ (Performance Qualification) vs OQ (Operational Qualification) Infographic
 libmatt.com
libmatt.com