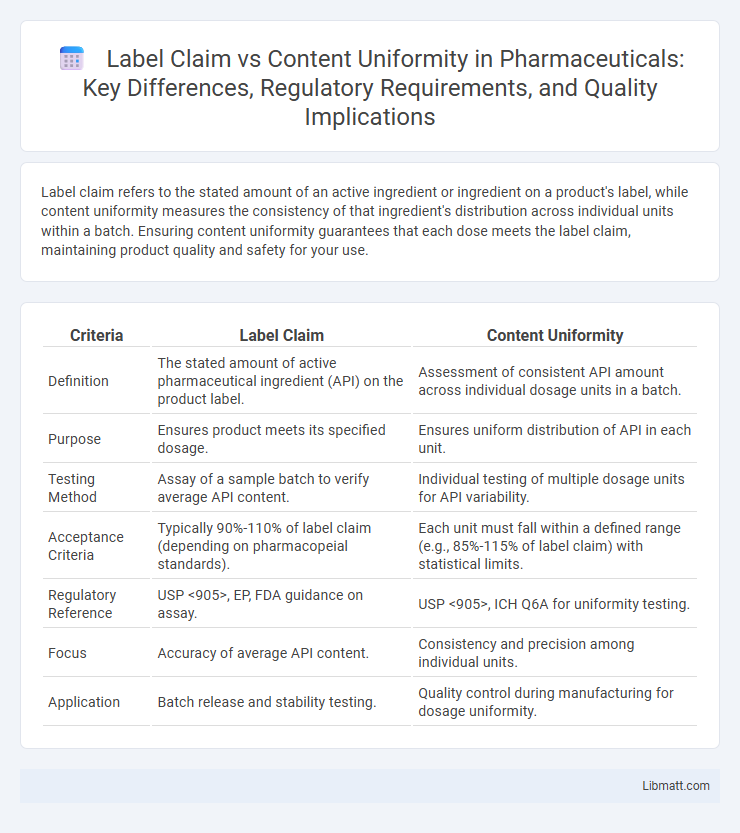
Label claim refers to the stated amount of an active ingredient or ingredient on a product's label, while content uniformity measures the consistency of that ingredient's distribution across individual units within a batch. Ensuring content uniformity guarantees that each dose meets the label claim, maintaining product quality and safety for your use.
Table of Comparison
| Criteria | Label Claim | Content Uniformity |
|---|---|---|
| Definition | The stated amount of active pharmaceutical ingredient (API) on the product label. | Assessment of consistent API amount across individual dosage units in a batch. |
| Purpose | Ensures product meets its specified dosage. | Ensures uniform distribution of API in each unit. |
| Testing Method | Assay of a sample batch to verify average API content. | Individual testing of multiple dosage units for API variability. |
| Acceptance Criteria | Typically 90%-110% of label claim (depending on pharmacopeial standards). | Each unit must fall within a defined range (e.g., 85%-115% of label claim) with statistical limits. |
| Regulatory Reference | USP <905>, EP, FDA guidance on assay. | USP <905>, ICH Q6A for uniformity testing. |
| Focus | Accuracy of average API content. | Consistency and precision among individual units. |
| Application | Batch release and stability testing. | Quality control during manufacturing for dosage uniformity. |
Introduction to Label Claim and Content Uniformity
Label claim represents the amount of active pharmaceutical ingredient (API) stated on a product's label, ensuring the medication delivers the intended dosage. Content uniformity assesses the consistency of API distribution across individual dosage units within a batch, confirming each unit meets specified potency limits. Both parameters are critical for quality control, regulatory compliance, and patient safety in pharmaceutical manufacturing.
Defining Label Claim in Pharmaceuticals
Label claim in pharmaceuticals refers to the specific amount of active ingredient stated on a product's packaging or label, ensuring the medication contains the correct potency for efficacy and safety. This claim must comply with regulatory standards set by authorities such as the FDA or EMA, requiring accurate quantification through rigorous testing. Content uniformity, by comparison, measures the consistency of the active ingredient across individual units within a batch, verifying each dose meets the label claim requirements.
Understanding Content Uniformity
Content uniformity ensures that each dosage unit contains the active ingredient within a specified range around the label claim, maintaining consistent potency throughout the batch. This parameter is critical for verifying that your pharmaceutical product delivers the intended therapeutic effect without significant variation. Regulatory guidelines, such as those from the USP, provide detailed testing methods to assess content uniformity and guarantee product quality.
Regulatory Requirements for Label Claim
Regulatory requirements for label claim mandate that the declared amount of an active ingredient on a pharmaceutical product's label must closely match the actual content within specified tolerance limits, typically set by agencies like the FDA or EMA. These regulations ensure consumer safety and product efficacy by requiring rigorous testing and verification through validated analytical methods. Compliance with label claim standards is essential for market approval, quality control, and avoiding legal liabilities.
Regulatory Guidelines for Content Uniformity
Regulatory guidelines for content uniformity ensure each dosage unit contains the active ingredient within a specified percentage of the label claim, typically 85-115% as per USP <905> standards. These guidelines require rigorous testing protocols to maintain consistent drug potency across batches, minimizing variability to guarantee patient safety and therapeutic efficacy. Your quality control processes must adhere to these standards to comply with FDA and international pharmacopeial requirements.
Key Differences Between Label Claim and Content Uniformity
Label claim specifies the declared amount of active pharmaceutical ingredient (API) stated on the product label, reflecting the target dosage per unit. Content uniformity measures the consistency of the API amount across individual dosage units within a batch, ensuring each unit meets predefined specifications. The key difference lies in label claim representing the intended dosage, while content uniformity verifies the actual variability and uniform distribution of API in each unit.
Methods for Assessing Label Claim
Methods for assessing label claim commonly include high-performance liquid chromatography (HPLC), UV-visible spectrophotometry, and titrimetric analysis, ensuring accurate measurement of the active pharmaceutical ingredient concentration. These analytical techniques provide quantitative data to verify that the labeled amount matches the actual content within specified acceptance criteria. Accuracy, precision, and specificity of the chosen method are critical for reliable label claim evaluation in pharmaceutical quality control.
Testing Procedures for Content Uniformity
Testing procedures for content uniformity involve precise analytical methods such as High Performance Liquid Chromatography (HPLC) or Near-Infrared Spectroscopy (NIR) to measure drug substance distribution within individual dosage units. According to USP guidelines, each unit must fall within a specified range of the labeled claim to ensure consistent potency and efficacy. Your pharmaceutical products undergo stringent content uniformity tests to maintain quality control and regulatory compliance.
Impact of Label Claim vs Content Uniformity on Drug Quality
Label claim ensures the drug contains the amount of active ingredient specified on the packaging, directly affecting the medication's efficacy and patient safety. Content uniformity tests verify consistent distribution of the active ingredient across dosage units, preventing dosage variability and potential therapeutic failure. Your drug's quality relies heavily on both accurate label claims and strict content uniformity to maintain regulatory compliance and patient trust.
Ensuring Compliance in Pharmaceutical Manufacturing
Label claim and content uniformity are critical parameters in pharmaceutical manufacturing to ensure dosage accuracy and regulatory compliance. Label claim verifies that each dosage unit contains the amount of active pharmaceutical ingredient (API) stated on the label, while content uniformity assesses the consistency of API distribution among individual units within a batch. Stringent adherence to USP <905> content uniformity testing and validation of analytical methods guarantees product quality, patient safety, and compliance with FDA and ICH guidelines.
Label claim vs content uniformity Infographic
 libmatt.com
libmatt.com