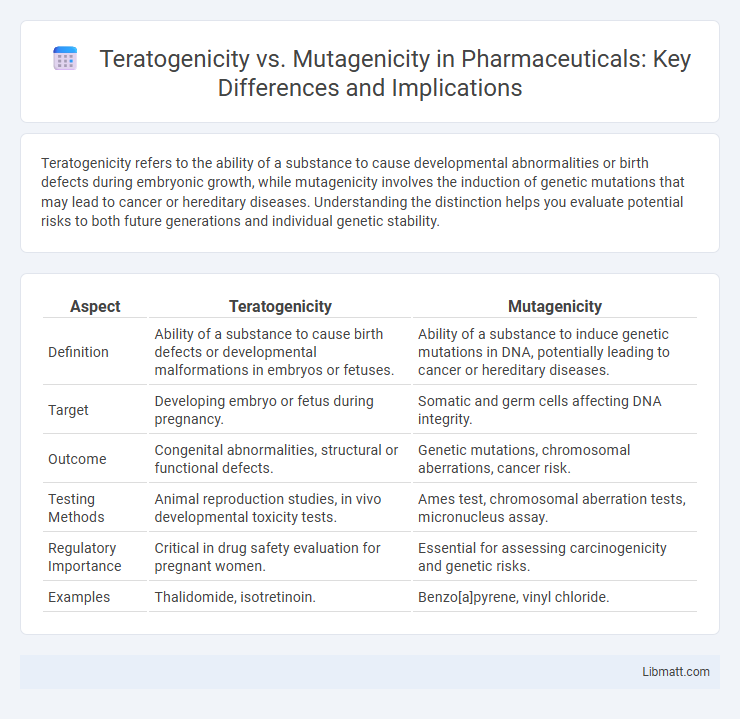
Teratogenicity refers to the ability of a substance to cause developmental abnormalities or birth defects during embryonic growth, while mutagenicity involves the induction of genetic mutations that may lead to cancer or hereditary diseases. Understanding the distinction helps you evaluate potential risks to both future generations and individual genetic stability.
Table of Comparison
| Aspect | Teratogenicity | Mutagenicity |
|---|---|---|
| Definition | Ability of a substance to cause birth defects or developmental malformations in embryos or fetuses. | Ability of a substance to induce genetic mutations in DNA, potentially leading to cancer or hereditary diseases. |
| Target | Developing embryo or fetus during pregnancy. | Somatic and germ cells affecting DNA integrity. |
| Outcome | Congenital abnormalities, structural or functional defects. | Genetic mutations, chromosomal aberrations, cancer risk. |
| Testing Methods | Animal reproduction studies, in vivo developmental toxicity tests. | Ames test, chromosomal aberration tests, micronucleus assay. |
| Regulatory Importance | Critical in drug safety evaluation for pregnant women. | Essential for assessing carcinogenicity and genetic risks. |
| Examples | Thalidomide, isotretinoin. | Benzo[a]pyrene, vinyl chloride. |
Introduction to Teratogenicity and Mutagenicity
Teratogenicity refers to the potential of certain substances or factors to cause developmental abnormalities or birth defects during embryonic or fetal development. Mutagenicity involves the ability of agents to induce genetic mutations, which can result in changes to DNA sequences. Understanding the differences between teratogenic and mutagenic effects is crucial for assessing risks to Your reproductive health and genetic integrity.
Defining Teratogenicity: Causes and Effects
Teratogenicity refers to the ability of certain substances, known as teratogens, to cause developmental malformations or congenital abnormalities during embryonic or fetal development. Common teratogens include drugs like thalidomide, environmental chemicals such as alcohol, and infectious agents like rubella virus, which disrupt normal cell differentiation and organ formation. The resulting effects range from minor structural defects to severe functional disabilities, emphasizing the critical importance of avoiding teratogenic exposures during pregnancy.
Understanding Mutagenicity: Mechanisms and Outcomes
Mutagenicity refers to the capacity of a substance or agent to induce genetic mutations by altering the DNA sequence in cells, potentially leading to hereditary changes and cancer development. The mechanisms underlying mutagenicity often involve direct DNA damage, such as base substitutions, insertions, deletions, or chromosomal aberrations caused by chemical agents or radiation exposure. Outcomes of mutagenicity include genetic instability, carcinogenesis, and transmission of genetic defects to offspring, making mutagenic evaluation critical in toxicology and drug safety assessment.
Key Differences between Teratogens and Mutagens
Teratogenicity refers to the ability of a substance to cause developmental abnormalities or birth defects during embryonic or fetal development, while mutagenicity involves genetic mutations that can alter DNA sequences in cells. Teratogens primarily affect prenatal development, leading to malformations or functional deficits, whereas mutagens induce heritable genetic changes that may result in cancer or inherited diseases. Your understanding of these mechanisms helps in assessing risks linked to exposure during pregnancy versus long-term genetic impacts.
Common Teratogenic Agents and Their Impact
Common teratogenic agents such as thalidomide, alcohol, and certain antiepileptic drugs disrupt fetal development, causing birth defects like limb malformations, fetal alcohol syndrome, and neural tube defects. These agents interfere with cellular differentiation and organogenesis during critical periods of embryonic growth, leading to structural anomalies and functional impairments. Understanding the specific mechanisms of these teratogens enables healthcare providers to minimize exposure risks and improve prenatal care outcomes.
Mutagenic Substances: Examples and Risks
Mutagenic substances such as benzene, formaldehyde, and ultraviolet radiation cause alterations in DNA sequences, increasing the risk of genetic mutations that can lead to cancer and hereditary defects. Exposure to mutagens in occupational settings or through environmental pollution elevates the likelihood of chromosomal abnormalities and gene mutations in somatic and germ cells. Risk mitigation involves strict regulatory guidelines and personal protective equipment to minimize DNA damage and subsequent health consequences.
Methods for Testing Teratogenicity and Mutagenicity
Testing teratogenicity primarily involves in vivo animal studies using rodent models and zebrafish embryos to observe developmental abnormalities, while in vitro techniques include embryonic stem cell assays and whole embryo culture systems. Mutagenicity is evaluated using assays such as the Ames test for bacterial reverse mutation, the micronucleus test for chromosomal damage, and the comet assay for DNA strand breaks in mammalian cells. Your safety assessments rely on combining these methods to identify potential risks of birth defects and genetic mutations effectively.
Human Health Implications: Teratogenicity vs Mutagenicity
Teratogenicity refers to the capability of substances to cause developmental malformations or birth defects during embryonic or fetal development, posing significant risks to prenatal human health. Mutagenicity involves the induction of genetic mutations that may lead to cancer, hereditary diseases, or genetic disorders affecting an individual's long-term health and reproductive outcomes. Understanding these distinct mechanisms is crucial for evaluating chemical safety and implementing regulatory measures to protect human populations from adverse health effects.
Regulatory Guidelines for Teratogenic and Mutagenic Agents
Regulatory guidelines for teratogenic and mutagenic agents require rigorous testing to assess potential risks to human health, ensuring compounds do not cause birth defects or genetic mutations. Agencies like the FDA and EMA mandate preclinical reproductive toxicity studies and genotoxicity assays to evaluate teratogenicity and mutagenicity before drug approval. Your compliance with these guidelines is essential to safeguard public health and meet legal standards during pharmaceutical development.
Future Research Directions in Teratogenicity and Mutagenicity
Future research in teratogenicity aims to identify specific molecular pathways and genetic susceptibilities that contribute to developmental abnormalities, enhancing early detection and prevention strategies. Advances in mutagenicity studies focus on high-throughput genomic screening and CRISPR-based models to better understand mutation mechanisms and their impacts on heritable diseases. Your understanding of these evolving research directions will be crucial for developing targeted therapies and improving public health risk assessments.
Teratogenicity vs Mutagenicity Infographic
 libmatt.com
libmatt.com