Metallic bonding involves the attraction between free-floating electrons and metal ions, creating strong, conductive, and malleable materials, while adhesive bonding relies on the adhesion forces between different surfaces using adhesives to join materials. Understanding the differences helps you choose the right technique for applications requiring electrical conductivity or durable, non-metallic joins.
Table of Comparison
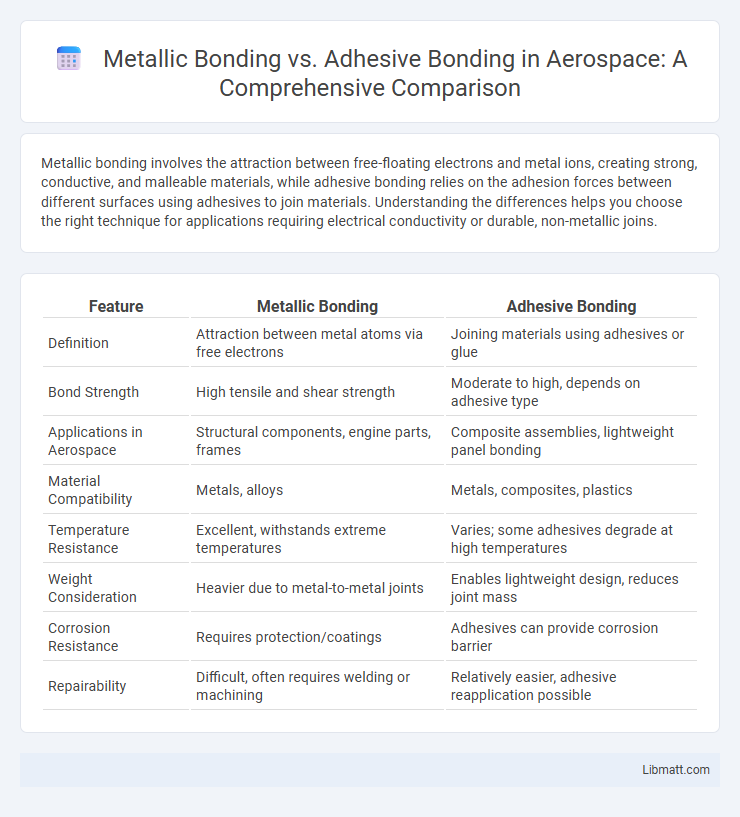
| Feature | Metallic Bonding | Adhesive Bonding |
|---|---|---|
| Definition | Attraction between metal atoms via free electrons | Joining materials using adhesives or glue |
| Bond Strength | High tensile and shear strength | Moderate to high, depends on adhesive type |
| Applications in Aerospace | Structural components, engine parts, frames | Composite assemblies, lightweight panel bonding |
| Material Compatibility | Metals, alloys | Metals, composites, plastics |
| Temperature Resistance | Excellent, withstands extreme temperatures | Varies; some adhesives degrade at high temperatures |
| Weight Consideration | Heavier due to metal-to-metal joints | Enables lightweight design, reduces joint mass |
| Corrosion Resistance | Requires protection/coatings | Adhesives can provide corrosion barrier |
| Repairability | Difficult, often requires welding or machining | Relatively easier, adhesive reapplication possible |
Introduction to Metallic and Adhesive Bonding
Metallic bonding occurs when metal atoms share free electrons, creating a lattice of positive ions held together by a sea of delocalized electrons, resulting in high electrical conductivity and strength. Adhesive bonding relies on the adhesion between surfaces using an adhesive material, forming a strong bond through chemical, mechanical, or physical interactions. Your choice between metallic and adhesive bonding depends on factors like material compatibility, application requirements, and desired mechanical properties.
Fundamental Principles of Metallic Bonding
Metallic bonding arises from the electrostatic attraction between a lattice of positive metal ions and a sea of delocalized valence electrons, enabling high electrical and thermal conductivity. The shared electron cloud allows metal atoms to slide past each other without breaking bonds, providing metals with their characteristic malleability and ductility. Fundamental to this bonding type is the collective interaction among all metal atoms, which contrasts with the localized molecular interactions found in adhesive bonding.
Basics of Adhesive Bonding
Adhesive bonding relies on the chemical and physical interactions at the interface between two surfaces, creating a strong bond through the use of adhesives such as epoxies, acrylics, or polyurethane. This method emphasizes surface preparation, including cleaning and sometimes abrasion, to ensure optimal adhesion by promoting mechanical interlocking and molecular attraction. Your application benefits from adhesive bonding when joining dissimilar materials or when flexibility and stress distribution are critical factors.
Comparison of Atomic Structures
Metallic bonding features a lattice of positively charged metal ions surrounded by a sea of delocalized electrons, allowing for high electrical conductivity and malleability. Adhesive bonding involves the attraction between different materials through physical or chemical interactions at the surfaces, relying on molecular forces such as van der Waals forces, hydrogen bonds, or covalent bonds. Understanding these atomic structures helps you select the appropriate bonding method for applications requiring specific mechanical strength or electrical properties.
Strength and Durability: Metallic vs Adhesive Bonds
Metallic bonding exhibits superior strength and durability due to the delocalized electrons that create a strong lattice structure, allowing metals to withstand high stress and temperature variations. Adhesive bonding relies on surface interactions and chemical adhesives, which can degrade over time under environmental exposure, reducing long-term performance. While metallic bonds are inherently robust and maintain integrity in extreme conditions, adhesive bonds require careful surface preparation and environmental control to achieve comparable durability.
Applications in Industry and Manufacturing
Metallic bonding is extensively used in industries such as automotive and aerospace for creating strong, conductive joints in steel and aluminum components, enhancing structural integrity and electrical performance. Adhesive bonding finds significant application in electronics, aerospace, and construction, enabling the joining of dissimilar materials like composites and metals while providing flexibility and resistance to environmental factors. Both bonding methods optimize manufacturing processes by improving product durability, reducing weight, and enabling complex assembly designs.
Environmental Resistance and Chemical Stability
Metallic bonding exhibits excellent environmental resistance due to its strong electron sharing among metal atoms, which provides high chemical stability against oxidation and corrosion under various conditions. Adhesive bonding, depending on the adhesive type and substrate, can face challenges with environmental resistance, particularly when exposed to moisture, UV radiation, or chemical solvents that degrade the bond over time. Your choice between metallic and adhesive bonding should consider the specific environmental exposure and chemical stability requirements to ensure long-lasting performance.
Advantages and Disadvantages of Each Bonding Type
Metallic bonding offers high electrical and thermal conductivity, excellent strength, and durability but is limited by poor corrosion resistance and difficulty in joining dissimilar metals. Adhesive bonding provides versatility in joining different materials and distributes stress evenly but tends to have lower strength and susceptibility to environmental degradation such as moisture and temperature changes. The choice depends on application requirements like mechanical strength, environmental exposure, and material compatibility.
Selection Criteria for Engineering Applications
Metallic bonding offers superior electrical conductivity, thermal resistance, and mechanical strength, making it ideal for applications requiring durability and efficient energy transfer. Adhesive bonding provides versatility in joining dissimilar materials and enables stress distribution over larger areas, which is beneficial in lightweight or composite structures. Your selection criteria should prioritize factors like load conditions, environmental exposure, material compatibility, and repairability to determine the most suitable bonding method for engineering applications.
Future Trends in Bonding Technologies
Future trends in bonding technologies emphasize the development of hybrid bonding methods that combine metallic bonding's high electrical conductivity and mechanical strength with adhesive bonding's ability to join dissimilar materials and provide environmental resistance. Advances in nanotechnology and surface engineering enhance metallic bonding by improving bond durability and corrosion resistance, while bio-based and smart adhesives are being designed for sustainable and self-healing applications. Integration of these technologies aims to create multifunctional bonds tailored for aerospace, automotive, and electronics industries, facilitating lighter, stronger, and more adaptable materials.
Metallic bonding vs Adhesive bonding Infographic
 libmatt.com
libmatt.com