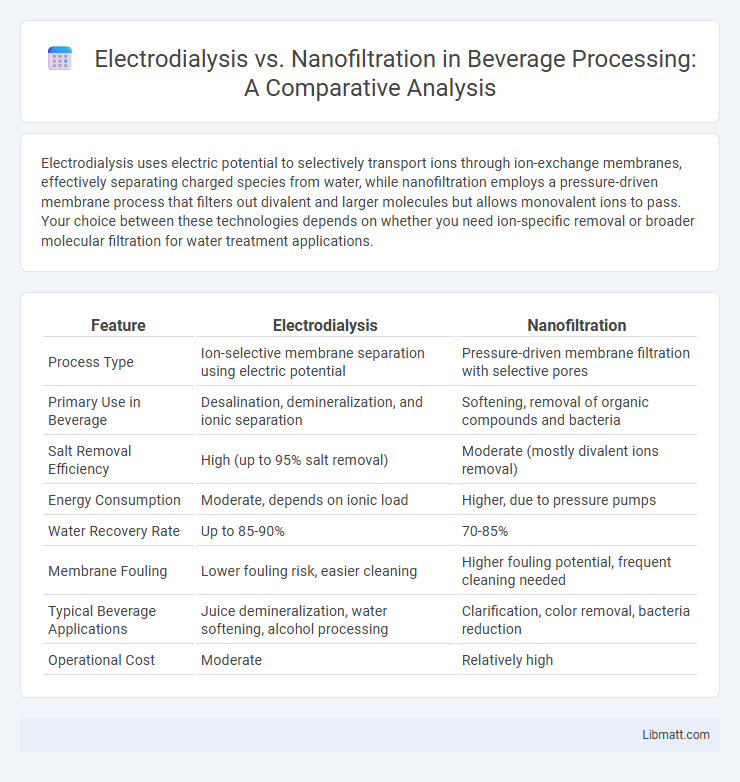
Electrodialysis uses electric potential to selectively transport ions through ion-exchange membranes, effectively separating charged species from water, while nanofiltration employs a pressure-driven membrane process that filters out divalent and larger molecules but allows monovalent ions to pass. Your choice between these technologies depends on whether you need ion-specific removal or broader molecular filtration for water treatment applications.
Table of Comparison
| Feature | Electrodialysis | Nanofiltration |
|---|---|---|
| Process Type | Ion-selective membrane separation using electric potential | Pressure-driven membrane filtration with selective pores |
| Primary Use in Beverage | Desalination, demineralization, and ionic separation | Softening, removal of organic compounds and bacteria |
| Salt Removal Efficiency | High (up to 95% salt removal) | Moderate (mostly divalent ions removal) |
| Energy Consumption | Moderate, depends on ionic load | Higher, due to pressure pumps |
| Water Recovery Rate | Up to 85-90% | 70-85% |
| Membrane Fouling | Lower fouling risk, easier cleaning | Higher fouling potential, frequent cleaning needed |
| Typical Beverage Applications | Juice demineralization, water softening, alcohol processing | Clarification, color removal, bacteria reduction |
| Operational Cost | Moderate | Relatively high |
Introduction to Electrodialysis and Nanofiltration
Electrodialysis utilizes an electric potential to selectively separate ions through ion-exchange membranes, making it highly effective for desalination and wastewater treatment. Nanofiltration employs semi-permeable membranes with pore sizes between 1 to 10 nanometers, effectively removing divalent and larger monovalent ions while allowing smaller molecules and water to pass. Both technologies serve distinct water purification roles, with electrodialysis excelling in ion-specific separation and nanofiltration providing broader filtration for organic compounds and particulates.
Core Principles of Electrodialysis
Electrodialysis operates by applying an electric potential to selectively transport ions through ion-exchange membranes, separating charged species from water based on their ionic charge and mobility. The process typically involves alternating cation and anion exchange membranes that create compartments for ion removal, making it effective for desalination and ion recovery. This method is energy-efficient for separating salts and minerals from water compared to pressure-driven membrane techniques like nanofiltration.
Fundamentals of Nanofiltration Technology
Nanofiltration technology employs semi-permeable membranes with pore sizes typically ranging from 1 to 10 nanometers, enabling selective separation of divalent and larger monovalent ions from water. This process operates under moderate pressure, making it energy-efficient for removing contaminants like hardness, organic compounds, and certain salts without completely desalinating the water. Your water treatment choice hinges on nanofiltration's ability to target specific solutes while maintaining beneficial minerals, contrasting with electrodialysis which uses electrical potential to separate ions based on charge.
Key Differences Between Electrodialysis and Nanofiltration
Electrodialysis uses an electrical potential to selectively transport ions through ion-exchange membranes, making it ideal for desalination and ion separation in water treatment. Nanofiltration relies on a semipermeable membrane with pore sizes around 1-10 nanometers that selectively retains divalent and larger molecules while allowing monovalent ions to pass, commonly used for softening and removing organic compounds. The key differences lie in their separation mechanisms--electrodialysis employs electrical driving forces targeting ionic species, whereas nanofiltration uses pressure-driven membrane filtration focusing on molecular size and charge exclusion.
Comparative Advantages of Electrodialysis
Electrodialysis offers superior ion selectivity and energy efficiency compared to nanofiltration, making it ideal for applications requiring precise desalination or ion removal, such as brackish water treatment and industrial wastewater recycling. Its ability to separate ions based on charge without high pressure reduces operational costs and extends membrane lifespan. Your water treatment process can benefit from electrodialysis when targeting specific ion removal with minimal chemical use and lower environmental impact.
Benefits and Limitations of Nanofiltration
Nanofiltration offers efficient removal of divalent and larger monovalent ions, organic molecules, and pathogens, making it ideal for water softening and partial desalination with lower energy consumption than reverse osmosis. However, nanofiltration membranes have limitations, such as sensitivity to fouling, limited rejection of small monovalent salts, and the need for pre-treatment to prevent membrane damage. Your choice between electrodialysis and nanofiltration depends on water composition, treatment goals, and operational costs, as nanofiltration excels in selective separation but may require more maintenance.
Typical Applications: Electrodialysis vs Nanofiltration
Electrodialysis is predominantly used for desalination, wastewater treatment, and the recovery of valuable salts in industries such as food processing and pharmaceuticals. Nanofiltration excels in water softening, removal of divalent and larger monovalent ions, and purification of drinking water, particularly in beverage production and dairy processing. Both techniques offer selective ion removal but differ in operational scope, with electrodialysis suited for ion exchange applications and nanofiltration ideal for partial separation and organic compound retention.
Performance Metrics: Efficiency and Selectivity
Electrodialysis offers high ion selectivity and energy efficiency for separating charged species, making it ideal for desalination and salt recovery in brackish water treatment. Nanofiltration provides superior selectivity for divalent and larger molecules while efficiently removing organic compounds and pathogens, with moderate energy consumption. Your choice depends on the targeted contaminants and balance between separation precision and operational cost.
Cost Analysis and Energy Consumption
Electrodialysis typically incurs higher initial capital costs due to specialized membrane stacks but offers lower operational energy consumption for high-salinity water treatment compared to nanofiltration, which demands more frequent membrane replacements and higher pressures. Nanofiltration systems often have reduced capital expenditure but consume more energy because they operate at higher pressures to force water through tighter membranes, increasing operational costs over time. Cost analysis reveals that electrodialysis is more cost-effective for brackish water desalination, while nanofiltration suits lower salinity feedwaters despite higher energy consumption.
Choosing the Right Technology for Your Needs
Electrodialysis offers efficient ion separation and desalination ideal for applications requiring selective salt removal and energy savings, while nanofiltration excels in removing organic molecules, divalent salts, and pathogens, making it suitable for water softening and contaminant reduction. Your choice depends on factors such as water composition, treatment goals, operational costs, and desired output quality. Evaluating these parameters ensures you select the technology that best matches your specific water treatment requirements.
Electrodialysis vs nanofiltration Infographic
 libmatt.com
libmatt.com