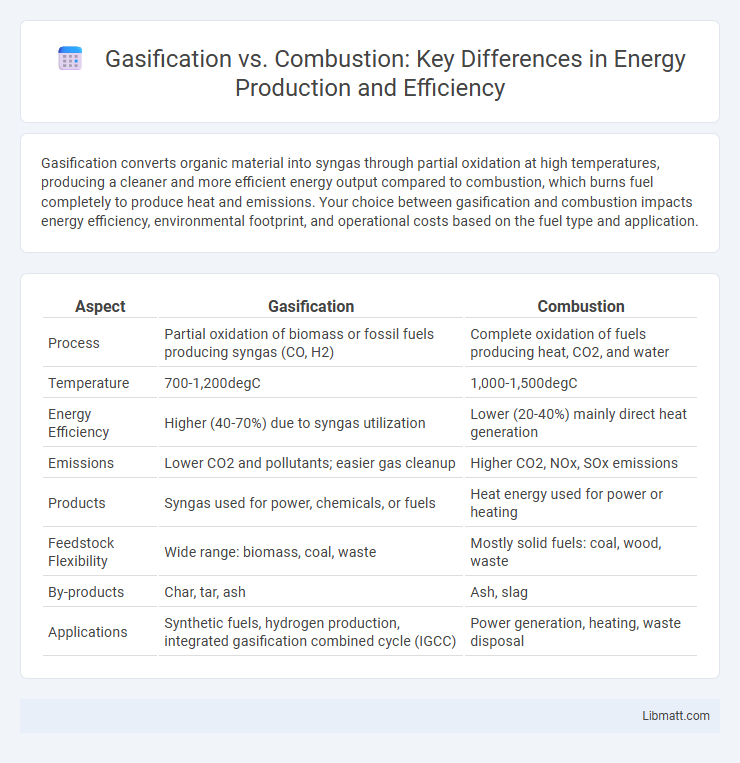
Gasification converts organic material into syngas through partial oxidation at high temperatures, producing a cleaner and more efficient energy output compared to combustion, which burns fuel completely to produce heat and emissions. Your choice between gasification and combustion impacts energy efficiency, environmental footprint, and operational costs based on the fuel type and application.
Table of Comparison
| Aspect | Gasification | Combustion |
|---|---|---|
| Process | Partial oxidation of biomass or fossil fuels producing syngas (CO, H2) | Complete oxidation of fuels producing heat, CO2, and water |
| Temperature | 700-1,200degC | 1,000-1,500degC |
| Energy Efficiency | Higher (40-70%) due to syngas utilization | Lower (20-40%) mainly direct heat generation |
| Emissions | Lower CO2 and pollutants; easier gas cleanup | Higher CO2, NOx, SOx emissions |
| Products | Syngas used for power, chemicals, or fuels | Heat energy used for power or heating |
| Feedstock Flexibility | Wide range: biomass, coal, waste | Mostly solid fuels: coal, wood, waste |
| By-products | Char, tar, ash | Ash, slag |
| Applications | Synthetic fuels, hydrogen production, integrated gasification combined cycle (IGCC) | Power generation, heating, waste disposal |
Introduction to Gasification and Combustion
Gasification and combustion are two distinct thermochemical processes used for energy production from carbon-based materials. Gasification converts organic materials into syngas--a mixture of carbon monoxide, hydrogen, and carbon dioxide--under limited oxygen conditions, offering higher efficiency and cleaner emissions. Combustion involves the complete oxidation of fuel in excess oxygen, producing heat, carbon dioxide, and water vapor, commonly used in traditional power generation and industrial applications.
Core Principles: How Gasification Works
Gasification converts carbon-based materials into synthesis gas (syngas) by reacting them with a controlled amount of oxygen or steam at high temperatures, typically between 700degC and 1,500degC. This process occurs in a low-oxygen environment, preventing complete combustion and allowing the fuel to undergo pyrolysis, oxidation, and reduction reactions that break down complex molecules into hydrogen, carbon monoxide, and methane. Gasification is highly efficient for producing cleaner energy and chemical feedstocks compared to traditional combustion, which burns fuel in excess oxygen to produce heat and carbon dioxide.
Core Principles: Understanding Combustion
Combustion is a chemical reaction that occurs when a fuel reacts rapidly with oxygen, producing heat, light, carbon dioxide, and water vapor. The core principles of combustion involve the ignition temperature, fuel-to-oxygen ratio, and the presence of sufficient activation energy to sustain a continuous exothermic reaction. Efficient combustion processes optimize fuel conversion and minimize pollutant formation through precise control of airflow and temperature.
Key Differences Between Gasification and Combustion
Gasification converts carbon-based materials into syngas through partial oxidation at high temperatures, producing mainly hydrogen, carbon monoxide, and methane, while combustion involves complete oxidation, producing carbon dioxide, water, and heat. Gasification operates in an oxygen-limited environment, resulting in higher energy efficiency and lower emissions of pollutants like NOx and SOx compared to combustion. The key distinction lies in the chemical process and output, with gasification yielding a versatile fuel gas and combustion primarily generating heat for immediate energy use.
Energy Efficiency: Gasification vs. Combustion
Gasification typically achieves higher energy efficiency than combustion by converting carbon-based materials into syngas, which can be used for electricity, heat, or fuel production with less energy loss. Combustion directly burns fuel, releasing energy primarily as heat while producing more emissions and lower fuel-to-energy conversion efficiency. The efficiency of gasification systems can exceed 70%, whereas conventional combustion systems often operate between 20-40% efficiency.
Environmental Impact Comparison
Gasification produces fewer pollutants and lower greenhouse gas emissions compared to combustion due to its partial oxidation process operating at lower temperatures. The syngas generated through gasification can be cleaned and utilized more efficiently, significantly reducing particulate matter, NOx, and SOx emissions. Conversely, combustion releases higher volumes of CO2 and toxic pollutants directly into the atmosphere, contributing more substantially to air pollution and climate change.
Feedstock Flexibility and Output Products
Gasification offers greater feedstock flexibility by converting a wide range of materials, including biomass, coal, and waste, into syngas rich in hydrogen and carbon monoxide, which can be used for power generation or chemical production. Combustion primarily burns homogeneous fuels like coal or natural gas to produce heat and electricity, with limited versatility in feedstock types. Your choice between these technologies impacts the diversity of usable raw materials and the variety of valuable energy or chemical outputs generated.
Industrial Applications: Where Each Method Excels
Gasification excels in industrial applications requiring synthesis gas production for chemicals, fertilizers, and liquid fuels, offering higher thermal efficiency and lower emissions compared to combustion. Combustion is widely used in power generation and waste incineration due to its simplicity, high energy release, and established technology for direct heat and electricity production. Industries such as steelmaking and refining benefit from gasification's ability to convert carbonaceous materials into valuable feedstocks, while combustion dominates in traditional boiler-based energy systems.
Challenges and Limitations of Both Technologies
Gasification faces challenges such as tar formation, ash disposal, and complex feedstock requirements, which impact efficiency and operational stability. Combustion is limited by higher emissions of pollutants like NOx and CO2, as well as lower energy conversion efficiency compared to gasification. Both technologies require advanced control systems to optimize performance and meet environmental regulations.
Future Trends in Gasification and Combustion
Future trends in gasification emphasize advanced syngas cleaning technologies and integration with carbon capture and storage (CCS) to enhance environmental sustainability. Combustion advancements focus on improving efficiency through ultra-low NOx burners and oxy-fuel combustion to reduce pollutant emissions. Your choice between these processes can be guided by evolving regulatory standards and the increasing demand for cleaner energy solutions.
Gasification vs Combustion Infographic
 libmatt.com
libmatt.com