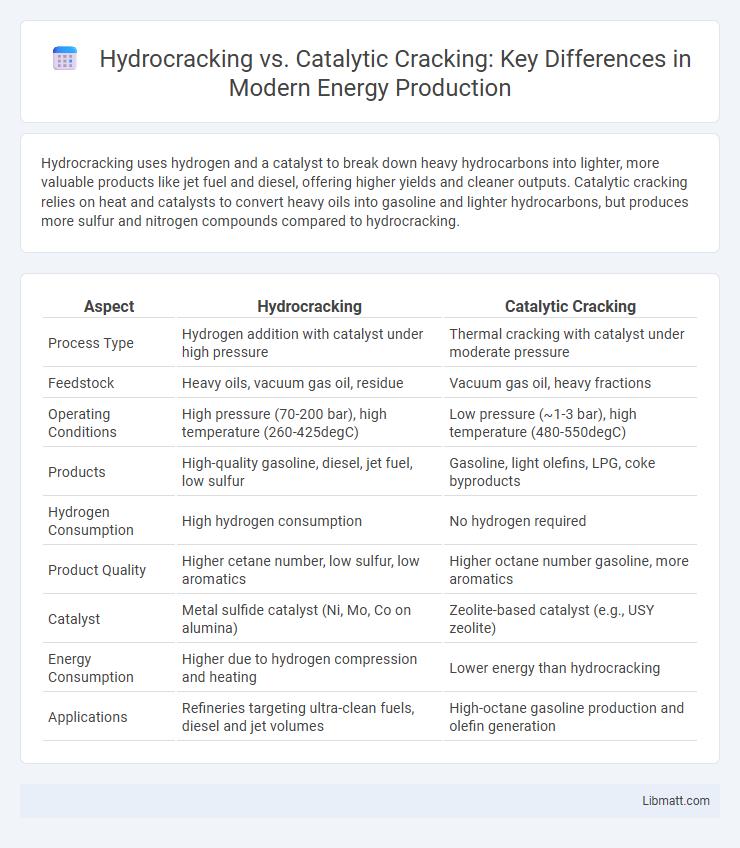
Hydrocracking uses hydrogen and a catalyst to break down heavy hydrocarbons into lighter, more valuable products like jet fuel and diesel, offering higher yields and cleaner outputs. Catalytic cracking relies on heat and catalysts to convert heavy oils into gasoline and lighter hydrocarbons, but produces more sulfur and nitrogen compounds compared to hydrocracking.
Table of Comparison
| Aspect | Hydrocracking | Catalytic Cracking |
|---|---|---|
| Process Type | Hydrogen addition with catalyst under high pressure | Thermal cracking with catalyst under moderate pressure |
| Feedstock | Heavy oils, vacuum gas oil, residue | Vacuum gas oil, heavy fractions |
| Operating Conditions | High pressure (70-200 bar), high temperature (260-425degC) | Low pressure (~1-3 bar), high temperature (480-550degC) |
| Products | High-quality gasoline, diesel, jet fuel, low sulfur | Gasoline, light olefins, LPG, coke byproducts |
| Hydrogen Consumption | High hydrogen consumption | No hydrogen required |
| Product Quality | Higher cetane number, low sulfur, low aromatics | Higher octane number gasoline, more aromatics |
| Catalyst | Metal sulfide catalyst (Ni, Mo, Co on alumina) | Zeolite-based catalyst (e.g., USY zeolite) |
| Energy Consumption | Higher due to hydrogen compression and heating | Lower energy than hydrocracking |
| Applications | Refineries targeting ultra-clean fuels, diesel and jet volumes | High-octane gasoline production and olefin generation |
Introduction to Hydrocracking and Catalytic Cracking
Hydrocracking and catalytic cracking are essential refining processes used to convert heavy petroleum fractions into lighter, more valuable products like gasoline, diesel, and jet fuel. Hydrocracking involves hydrogen gas and a catalyst to break large hydrocarbons under high pressure and temperature, producing cleaner fuels with lower sulfur content. Catalytic cracking uses a solid catalyst at moderate pressure to crack heavy hydrocarbons, maximizing gasoline yield by favoring branched hydrocarbons and olefins formation.
Fundamental Principles of Hydrocracking
Hydrocracking operates by breaking down heavy hydrocarbons into lighter, more valuable products using hydrogen and a bifunctional catalyst composed of acidic and metallic sites. This process enhances product quality by saturating olefins and removing sulfur and nitrogen compounds, resulting in cleaner fuels. Your refinery can achieve higher yields of jet fuel, diesel, and gasoline while meeting stringent environmental regulations through precise temperature and pressure control.
Core Mechanisms of Catalytic Cracking
Catalytic cracking involves breaking down large hydrocarbon molecules into smaller, more valuable ones like gasoline and diesel through the use of a solid acid catalyst, typically zeolites. This process relies on the generation of carbocations to cleave carbon-carbon bonds, enhancing selectivity towards branched hydrocarbons that improve fuel quality. Your refinery operations benefit from the efficiency of this mechanism, which operates at moderate temperatures and atmospheric pressure to maximize yield and product stability.
Feedstock Requirements and Suitability
Hydrocracking requires feedstocks with higher hydrogen content and lower sulfur levels, typically favoring heavier, high-boiling petroleum fractions like vacuum gas oils and residuum for conversion into diesel and jet fuels. Catalytic cracking processes are more flexible with feedstock, efficiently handling heavier vacuum gas oils and residual oils with higher sulfur and nitrogen content, mainly producing gasoline and lighter hydrocarbons. The choice between hydrocracking and catalytic cracking depends heavily on feedstock quality, where hydrocracking suits cleaner, heavier feeds requiring high-value middle distillates, while catalytic cracking excels in upgrading heavier, more contaminated feedstocks into lighter fuel products.
Key Process Conditions and Operation
Hydrocracking operates under high pressure (typically 70-200 bar) and moderate temperatures (260-425degC) using hydrogen gas and a bifunctional catalyst, enabling the breaking of heavy hydrocarbons into lighter, saturated products with minimal coke formation. Catalytic cracking runs at atmospheric or slightly elevated pressure (1-3 bar) with higher temperatures (450-750degC) and a solid acid catalyst, favoring the cleavage of large hydrocarbon molecules into gasoline-range products but producing more olefins and coke. Your choice between hydrocracking and catalytic cracking depends on feedstock type and desired output, balancing hydrogen consumption, operating costs, and product quality.
Product Yield and Quality Comparison
Hydrocracking produces higher yields of premium products such as diesel, jet fuel, and naphtha with superior sulfur removal and improved cetane numbers, enhancing overall fuel quality. Catalytic cracking primarily generates gasoline with moderate diesel output but often results in higher sulfur content and lower octane ratings compared to hydrocracking. Your choice between these processes depends on the desired product slate and fuel quality requirements, with hydrocracking favored for cleaner, more energy-dense fuels.
Environmental Impact and Byproduct Analysis
Hydrocracking produces fewer sulfur compounds and nitrogen oxides compared to catalytic cracking, resulting in lower emissions and reduced environmental pollution. The process yields higher-quality, cleaner-burning fuels with minimal production of aromatic hydrocarbons and coke byproducts, which decreases particulate matter and greenhouse gas emissions. Catalytic cracking generates more environmentally harmful byproducts such as coke and sulfur compounds, necessitating additional treatment and disposal measures to mitigate its ecological footprint.
Catalyst Types and Lifespan
Hydrocracking uses bifunctional catalysts that combine acidic sites with metal sites, typically nickel-molybdenum or cobalt-molybdenum on an alumina base, which enhance hydrogenation and cracking reactions, resulting in longer catalyst lifespan due to reduced coking. Catalytic cracking relies on zeolite-based catalysts, rich in acidic sites, that promote carbonium ion mechanisms but have shorter lifespans because of faster deactivation from coke deposition. Your choice between these processes impacts catalyst management strategies, as hydrocracking catalysts require periodic regeneration and replacement less frequently than catalytic cracking catalysts.
Industrial Applications and Market Demand
Hydrocracking is widely utilized in producing high-quality diesel, jet fuel, and base oils, meeting stringent environmental regulations and growing demand for cleaner fuels in transportation and industrial sectors. Catalytic cracking remains essential for maximizing gasoline yield and producing lighter hydrocarbons, supporting the vast gasoline market and petrochemical feedstocks. Market trends indicate a shift towards hydrocracking due to increasing emphasis on fuel quality and emission control, while catalytic cracking maintains relevance in regions with high gasoline consumption.
Economic Considerations and Future Trends
Hydrocracking offers higher yields of premium products like diesel and jet fuel, which can justify its higher operational costs compared to catalytic cracking, making it economically favorable for refineries targeting quality output. Catalytic cracking remains a cost-effective choice for producing gasoline and lighter hydrocarbons, benefiting from lower hydrogen requirements and simpler processing conditions. Future trends indicate growing investments in hydrocracking due to stricter environmental regulations and increasing demand for cleaner fuels, while advancements in catalyst technology aim to enhance efficiency for both processes.
Hydrocracking vs Catalytic Cracking Infographic
 libmatt.com
libmatt.com