Aseptic sterilization involves sterilizing components separately before assembly in a sterile environment, ensuring minimal contamination risk during the process. Terminal sterilization sterilizes the final product in its container, offering a more robust microbial kill but requiring the product to withstand harsher conditions.
Table of Comparison
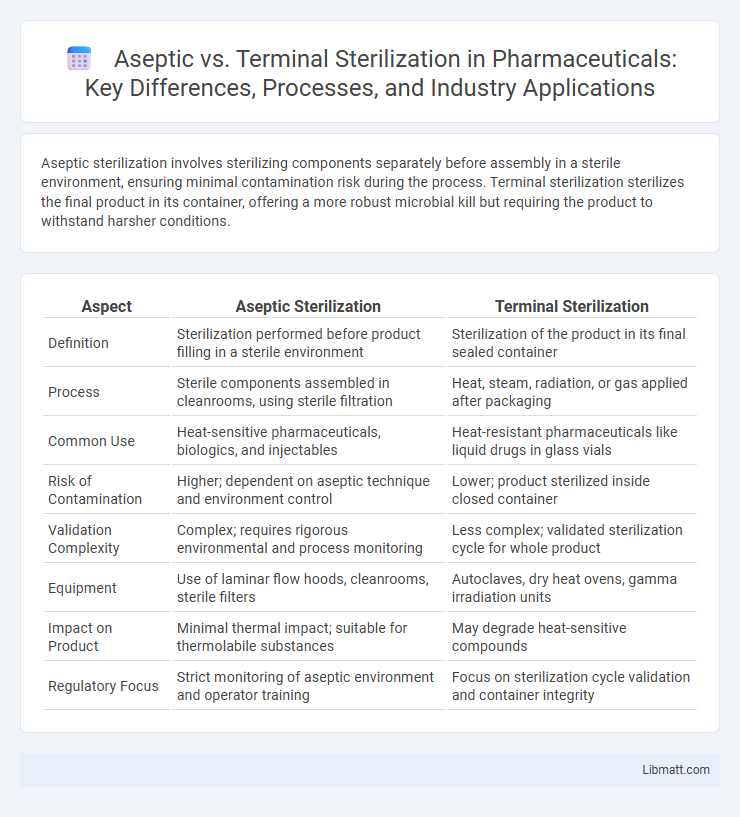
| Aspect | Aseptic Sterilization | Terminal Sterilization |
|---|---|---|
| Definition | Sterilization performed before product filling in a sterile environment | Sterilization of the product in its final sealed container |
| Process | Sterile components assembled in cleanrooms, using sterile filtration | Heat, steam, radiation, or gas applied after packaging |
| Common Use | Heat-sensitive pharmaceuticals, biologics, and injectables | Heat-resistant pharmaceuticals like liquid drugs in glass vials |
| Risk of Contamination | Higher; dependent on aseptic technique and environment control | Lower; product sterilized inside closed container |
| Validation Complexity | Complex; requires rigorous environmental and process monitoring | Less complex; validated sterilization cycle for whole product |
| Equipment | Use of laminar flow hoods, cleanrooms, sterile filters | Autoclaves, dry heat ovens, gamma irradiation units |
| Impact on Product | Minimal thermal impact; suitable for thermolabile substances | May degrade heat-sensitive compounds |
| Regulatory Focus | Strict monitoring of aseptic environment and operator training | Focus on sterilization cycle validation and container integrity |
Introduction to Sterilization Methods
Aseptic sterilization involves the sterilization of individual components before they are assembled in a sterile environment, ensuring the prevention of microbial contamination throughout the manufacturing process. Terminal sterilization, on the other hand, sterilizes the final product within its sealed container, offering a highly reliable method for eliminating contaminants after packaging. Choosing between aseptic and terminal sterilization depends on the product's heat sensitivity, packaging integrity, and your specific sterilization requirements.
Definition of Aseptic Sterilization
Aseptic sterilization refers to the process of sterilizing pharmaceutical products by separately sterilizing the product and its packaging components, followed by assembling them in a sterile environment to prevent contamination. This method uses filtration or heat to sterilize the product before filling it into sterilized containers under controlled sterile conditions. It is commonly employed for heat-sensitive drugs and biologics to maintain product integrity and sterility.
Definition of Terminal Sterilization
Terminal sterilization is a sterilization process where products are sealed in their final containers and then exposed to sterilizing agents like steam, ethylene oxide, or radiation to eliminate all viable microorganisms. This method ensures the highest level of sterility assurance by sterilizing the finished product, reducing the risk of contamination post-process. Your choice between aseptic and terminal sterilization depends on the product's heat sensitivity and packaging requirements.
Key Differences Between Aseptic and Terminal Sterilization
Aseptic sterilization involves sterilizing components separately and then assembling them in a sterile environment to prevent contamination, whereas terminal sterilization sterilizes the product within its final sealed container. Aseptic processing is essential for heat-sensitive products that cannot withstand high temperatures used in terminal sterilization, which typically employs steam, ethylene oxide, or radiation. The primary difference lies in contamination risk; terminal sterilization offers a lower contamination risk due to sterilization after packaging, while aseptic processing requires stricter environmental controls to maintain sterility.
Process Overview: Aseptic Sterilization
Aseptic sterilization involves sterilizing pharmaceutical components separately before assembling them in a sterile environment, ensuring the final product remains free from microbial contamination. This process typically includes sterilizing the product, container, and closure using methods such as filtration, steam, or heat, followed by filling under controlled cleanroom conditions. Maintaining rigorous environmental controls and validated sterilization procedures is critical to uphold product sterility and efficacy.
Process Overview: Terminal Sterilization
Terminal sterilization involves the final sterilization of medical products within their sealed containers using methods such as steam, ethylene oxide, or radiation. This process ensures microbial inactivation by exposing the entire product to sterilizing conditions after packaging, which reduces the risk of contamination during handling. Terminal sterilization is preferred for its reliability, validated sterility assurance levels, and suitability for heat- and moisture-stable pharmaceuticals and devices.
Applications in Pharmaceutical Manufacturing
Aseptic sterilization is widely used in pharmaceutical manufacturing for heat-sensitive products such as vaccines, biologics, and injectable solutions that cannot withstand high temperatures. Terminal sterilization is preferred for heat-stable pharmaceuticals, including aqueous solutions, suspensions, and some ophthalmic products, ensuring complete sterilization inside the sealed container. Your choice between aseptic and terminal sterilization depends on product formulation, thermal stability, and process feasibility to maintain sterility and efficacy.
Regulatory and Compliance Considerations
Regulatory agencies such as the FDA and EMA impose stringent guidelines for both aseptic and terminal sterilization processes to ensure product safety and efficacy. Aseptic processing requires comprehensive environmental monitoring, validated cleanroom practices, and rigorous operator training to comply with GMP standards. Terminal sterilization mandates validated sterilization cycles and sterility assurance levels (SAL), with regulatory audits emphasizing process validation and documentation adherence.
Advantages and Disadvantages of Each Method
Aseptic sterilization offers precise control over product quality and is ideal for heat-sensitive pharmaceuticals but involves complex validation and higher costs due to the need for sterile environments. Terminal sterilization ensures a robust kill step with a simpler process and lower contamination risk but can degrade heat-sensitive materials and is unsuitable for certain products. Choosing between them requires balancing sterility assurance levels with product stability and manufacturing feasibility.
Choosing the Right Sterilization Method
Choosing the right sterilization method depends on factors such as the product's heat sensitivity, packaging material, and sterility assurance level required. Aseptic sterilization involves separately sterilizing the product and packaging before filling in a sterile environment, ideal for heat-sensitive pharmaceuticals and biologics. Terminal sterilization sterilizes the product inside its final container, offering higher sterility assurance but requiring the product to tolerate high heat or radiation.
Aseptic vs Terminal sterilization Infographic
 libmatt.com
libmatt.com