Sterile filtration uses membrane filters with precise pore sizes to remove bacteria and microorganisms, ensuring the final product is free of contaminants, making it ideal for sterilizing liquids in pharmaceuticals and biotechnology. Depth filtration, on the other hand, captures particles within a thick, porous medium, effectively filtering larger volumes of fluids with high particle loads but without guaranteeing sterility.
Table of Comparison
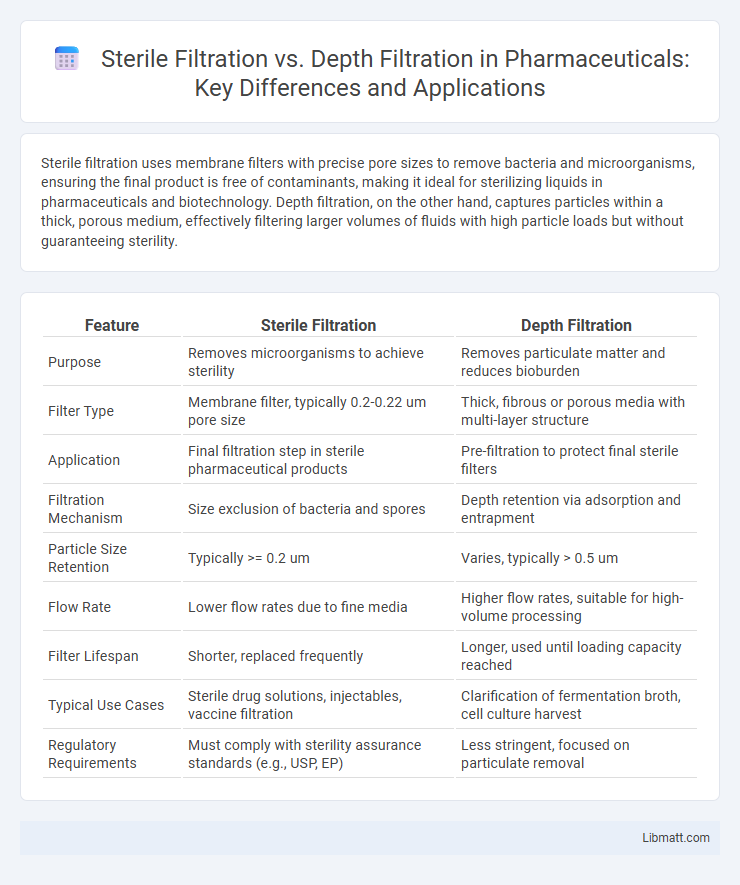
| Feature | Sterile Filtration | Depth Filtration |
|---|---|---|
| Purpose | Removes microorganisms to achieve sterility | Removes particulate matter and reduces bioburden |
| Filter Type | Membrane filter, typically 0.2-0.22 um pore size | Thick, fibrous or porous media with multi-layer structure |
| Application | Final filtration step in sterile pharmaceutical products | Pre-filtration to protect final sterile filters |
| Filtration Mechanism | Size exclusion of bacteria and spores | Depth retention via adsorption and entrapment |
| Particle Size Retention | Typically >= 0.2 um | Varies, typically > 0.5 um |
| Flow Rate | Lower flow rates due to fine media | Higher flow rates, suitable for high-volume processing |
| Filter Lifespan | Shorter, replaced frequently | Longer, used until loading capacity reached |
| Typical Use Cases | Sterile drug solutions, injectables, vaccine filtration | Clarification of fermentation broth, cell culture harvest |
| Regulatory Requirements | Must comply with sterility assurance standards (e.g., USP, EP) | Less stringent, focused on particulate removal |
Introduction to Filtration in Bioprocessing
Sterile filtration and depth filtration are essential techniques in bioprocessing to ensure product purity and safety by removing contaminants. Sterile filtration employs membrane filters with defined pore sizes, typically 0.22 microns, to eliminate microorganisms and achieve sterility in your final product. Depth filtration captures particulates throughout a thick filter matrix, offering high contaminant capacity and protection for subsequent sterile filtration steps.
Understanding Sterile Filtration
Sterile filtration uses membrane filters with pore sizes typically around 0.2 microns to remove microorganisms and ensure product sterility, making it essential in pharmaceutical and biotechnology applications. Unlike depth filtration, which relies on thick filter media to trap particles throughout the material, sterile filtration provides a defined barrier, preventing passage of bacteria and fungi while maintaining high flow rates and product integrity. This precise filtration method is crucial for terminal sterilization of heat-sensitive liquids, offering reliable microbial removal without compromising the product's quality or stability.
Overview of Depth Filtration
Depth filtration captures contaminants within a thick filter media, making it ideal for pre-filtration of liquids with high particulate loads. This method provides extended filter life and protects downstream sterile filters by removing larger particles and debris. Your filtration process benefits from improved efficiency and reduced risk of rapid clogging compared to sterile filtration alone.
Key Differences Between Sterile and Depth Filtration
Sterile filtration uses membrane filters with pore sizes typically around 0.2 microns, effectively removing microorganisms to ensure product sterility. Depth filtration relies on thick, porous media that trap particles throughout its matrix, excelling in removing larger debris and particulate matter but not guaranteeing sterility. Understanding these key differences helps you select the appropriate filtration method for protecting product integrity and meeting regulatory standards.
Applications of Sterile Filtration
Sterile filtration is widely used in pharmaceutical manufacturing to remove bacteria and other microorganisms from liquids, ensuring product sterility in injectable drugs, vaccines, and ophthalmic solutions. It is essential for heat-sensitive fluids that cannot undergo terminal sterilization, preserving the integrity of active ingredients. This method also finds applications in biotechnology for sterile media preparation and in the food and beverage industry to guarantee microbial safety without altering flavor or nutritional content.
Applications of Depth Filtration
Depth filtration is widely used in applications requiring the removal of high levels of particulates and contaminants from liquids, such as in the pharmaceutical, food and beverage, and water treatment industries. It effectively captures large volumes of suspended solids by trapping particles within a thick filtration medium, making it ideal for pre-filtration or clarification processes before sterile filtration. Your production process benefits from improved product clarity and extended lifespan of final sterilizing filters when incorporating depth filtration.
Performance Comparison: Efficiency and Throughput
Sterile filtration offers higher efficiency in removing microorganisms, ensuring superior sterility compared to depth filtration, which excels in handling higher throughput due to its thicker media and ability to trap larger particles. Depth filtration provides greater capacity for particulates, resulting in longer filter life and lower operating costs when processing fluids with high solids content. Your choice between sterile and depth filtration depends on balancing the need for microbial removal with desired filtration speed and volume capacity.
Filtration Mechanisms: How Each Method Works
Sterile filtration relies on membrane filters with precise pore sizes, typically 0.2 microns, physically removing bacteria and microorganisms to ensure a sterile output. Depth filtration functions through a thick matrix of fibrous or granular material that traps particles within its complex structure via mechanisms like adsorption, interception, and inertial impaction. Your choice depends on whether you need a defined microbial barrier or high particle-loading capacity with less filter clogging.
Selection Criteria: When to Use Sterile or Depth Filtration
Sterile filtration is ideal for applications requiring the removal of microorganisms and particles to achieve sterilization, especially in pharmaceutical and biotechnology processes where final product sterility is critical. Depth filtration is selected for pre-filtration or clarification to remove larger particulate matter and reduce load on downstream sterile filters, commonly used in food and beverage or chemical manufacturing. Key criteria include particle size, microbial load, process volume, and desired filtration efficiency to balance cost-effectiveness and product safety.
Future Trends in Filtration Technologies
Future trends in filtration technologies emphasize advanced materials and smart filtration systems enhancing both sterile filtration and depth filtration. Innovations such as nanofiber membranes and real-time monitoring sensors improve filtration efficiency, ensuring higher purity and reducing contamination risks in biopharmaceuticals and water treatment. Your filtration processes will benefit from these developments through increased reliability and cost-effectiveness, supporting evolving regulatory standards and sustainability goals.
Sterile filtration vs Depth filtration Infographic
 libmatt.com
libmatt.com