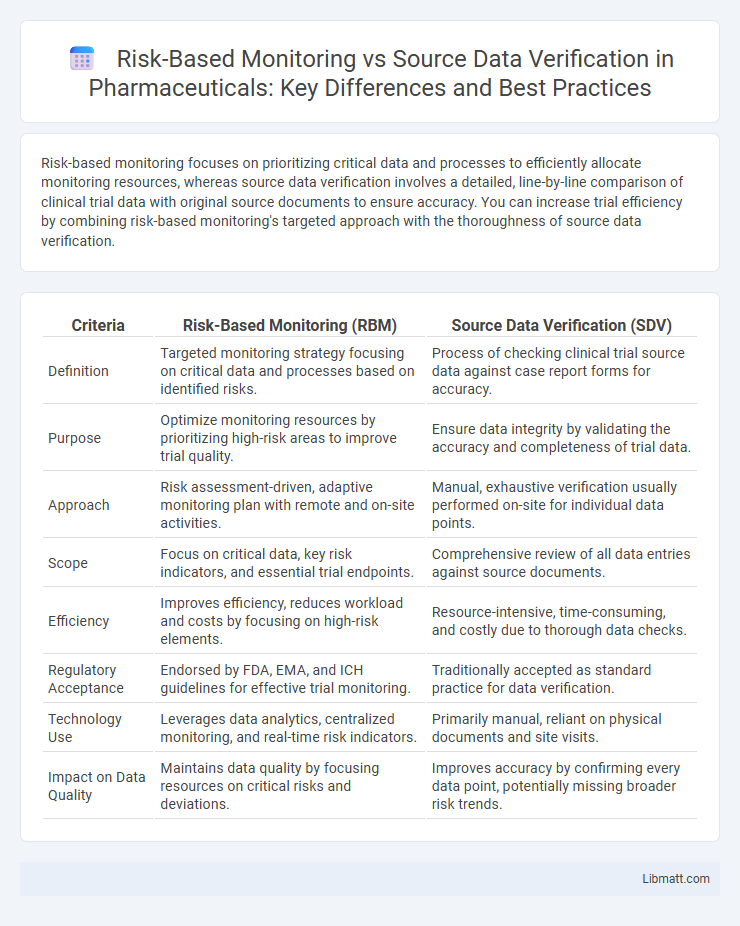
Risk-based monitoring focuses on prioritizing critical data and processes to efficiently allocate monitoring resources, whereas source data verification involves a detailed, line-by-line comparison of clinical trial data with original source documents to ensure accuracy. You can increase trial efficiency by combining risk-based monitoring's targeted approach with the thoroughness of source data verification.
Table of Comparison
| Criteria | Risk-Based Monitoring (RBM) | Source Data Verification (SDV) |
|---|---|---|
| Definition | Targeted monitoring strategy focusing on critical data and processes based on identified risks. | Process of checking clinical trial source data against case report forms for accuracy. |
| Purpose | Optimize monitoring resources by prioritizing high-risk areas to improve trial quality. | Ensure data integrity by validating the accuracy and completeness of trial data. |
| Approach | Risk assessment-driven, adaptive monitoring plan with remote and on-site activities. | Manual, exhaustive verification usually performed on-site for individual data points. |
| Scope | Focus on critical data, key risk indicators, and essential trial endpoints. | Comprehensive review of all data entries against source documents. |
| Efficiency | Improves efficiency, reduces workload and costs by focusing on high-risk elements. | Resource-intensive, time-consuming, and costly due to thorough data checks. |
| Regulatory Acceptance | Endorsed by FDA, EMA, and ICH guidelines for effective trial monitoring. | Traditionally accepted as standard practice for data verification. |
| Technology Use | Leverages data analytics, centralized monitoring, and real-time risk indicators. | Primarily manual, reliant on physical documents and site visits. |
| Impact on Data Quality | Maintains data quality by focusing resources on critical risks and deviations. | Improves accuracy by confirming every data point, potentially missing broader risk trends. |
Introduction to Clinical Trial Monitoring Methods
Risk-based monitoring (RBM) in clinical trials prioritizes oversight based on potential risks to patient safety and data integrity, optimizing resource allocation and enhancing trial efficiency. Source data verification (SDV) involves the detailed comparison of clinical data recorded in source documents with data entered into electronic case report forms, traditionally serving as a primary method for ensuring accuracy and compliance. Combining RBM with targeted SDV improves monitoring effectiveness by focusing efforts on critical data points and high-risk sites, aligning with regulatory guidelines from agencies like the FDA and EMA.
Defining Risk-Based Monitoring (RBM)
Risk-Based Monitoring (RBM) is a strategic approach that prioritizes clinical trial activities according to risk levels, optimizing resource allocation to ensure data quality and patient safety. Unlike Source Data Verification (SDV), which involves exhaustive, line-by-line checks against original records, RBM uses centralized analytics and targeted on-site visits to identify and address critical risks. Your clinical trial efficiency improves as RBM minimizes unnecessary data reviews while maintaining regulatory compliance and enhancing overall study integrity.
Understanding Source Data Verification (SDV)
Source Data Verification (SDV) is a critical process in clinical trials that ensures data accuracy by cross-checking trial data against the original source documents. It verifies the integrity of data collected from patient records, laboratory reports, and case report forms to maintain regulatory compliance and data reliability. Understanding SDV allows you to assess its role in the broader context of risk-based monitoring strategies, where targeted verification enhances efficiency without compromising data quality.
Key Differences Between RBM and SDV
Risk-based monitoring (RBM) prioritizes monitoring activities based on the level of risk associated with clinical trial sites and processes, optimizing resource allocation and focusing on critical data and procedures. Source data verification (SDV) involves the direct comparison of clinical trial data against original source documents to ensure accuracy and completeness, often performed extensively at the site level. Your clinical trial management benefits from RBM's strategic oversight, reducing reliance on exhaustive SDV and enhancing efficiency through targeted risk assessment.
Advantages of Risk-Based Monitoring
Risk-Based Monitoring (RBM) enhances clinical trial efficiency by prioritizing critical data and key risk areas, reducing the volume of unnecessary on-site visits compared to traditional Source Data Verification (SDV). RBM leverages real-time analytics and centralized data review, enabling early detection of potential issues and allowing more agile decision-making. By focusing your resources on high-risk sites and processes, RBM improves data quality and patient safety while lowering monitoring costs.
Limitations of Source Data Verification
Source Data Verification (SDV) often requires extensive manual effort, making it time-consuming and costly for clinical trials. It can miss broader trends and risks since SDV focuses narrowly on verifying individual data points rather than evaluating overall data quality or trial processes. Your monitoring strategy benefits from Risk-based Monitoring (RBM), which enhances efficiency by prioritizing critical data and key risk indicators over exhaustive SDV.
Impact on Data Quality and Patient Safety
Risk-based monitoring (RBM) enhances data quality and patient safety by targeting critical data points and high-risk sites, reducing unnecessary workload while maintaining oversight on essential trial parameters. Source data verification (SDV), though thorough, is resource-intensive and may overlook broader data trends impacting patient safety due to its focus on 100% data checking. Your clinical trial benefits more from RBM's strategic approach, which improves data integrity and patient safety through focused, risk-prioritized activities rather than exhaustive verification.
Regulatory Perspectives on RBM and SDV
Regulatory perspectives on risk-based monitoring (RBM) emphasize its efficiency in targeting critical trial parameters to enhance patient safety and data quality, as supported by agencies like the FDA and EMA. Source data verification (SDV), traditionally a cornerstone of monitoring, is now viewed as a component within RBM frameworks rather than a standalone requirement, promoting a shift away from 100% SDV. Your clinical trial oversight can benefit from aligning with these evolving regulatory guidelines, balancing RBM strategies with focused SDV to ensure compliance and optimize resource allocation.
Choosing the Right Monitoring Approach
Risk-based monitoring prioritizes critical data and processes by leveraging centralized data analytics to identify potential risks, enabling targeted oversight that enhances efficiency and resource allocation. Source data verification involves thorough, on-site cross-checking of clinical trial data against original records to ensure accuracy and compliance, but it is often resource-intensive and time-consuming. Selecting the optimal monitoring approach depends on the trial's complexity, risk profile, regulatory requirements, and available resources, balancing comprehensive data integrity with operational feasibility.
Future Trends in Clinical Trial Monitoring
Future trends in clinical trial monitoring emphasize the integration of risk-based monitoring (RBM) with advanced analytics and machine learning to enhance efficiency and data quality. Source data verification (SDV) is becoming more targeted, focusing on high-risk sites and critical data points rather than comprehensive 100% checks. Your clinical trial oversight will increasingly rely on hybrid approaches combining RBM, real-time data capture, and remote monitoring technologies to optimize resource use and ensure regulatory compliance.
Risk-based monitoring vs source data verification Infographic
 libmatt.com
libmatt.com