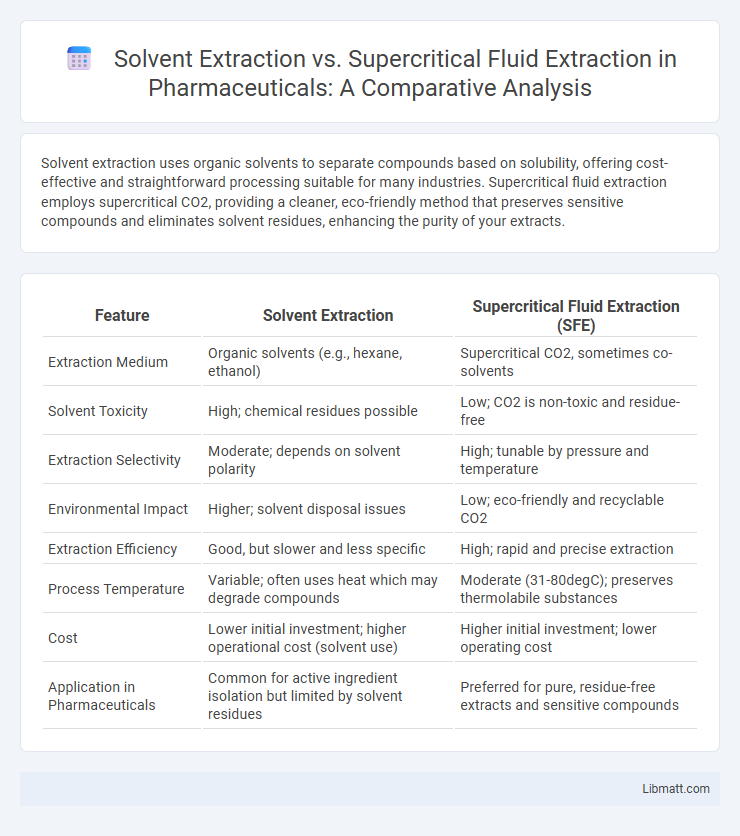
Solvent extraction uses organic solvents to separate compounds based on solubility, offering cost-effective and straightforward processing suitable for many industries. Supercritical fluid extraction employs supercritical CO2, providing a cleaner, eco-friendly method that preserves sensitive compounds and eliminates solvent residues, enhancing the purity of your extracts.
Table of Comparison
| Feature | Solvent Extraction | Supercritical Fluid Extraction (SFE) |
|---|---|---|
| Extraction Medium | Organic solvents (e.g., hexane, ethanol) | Supercritical CO2, sometimes co-solvents |
| Solvent Toxicity | High; chemical residues possible | Low; CO2 is non-toxic and residue-free |
| Extraction Selectivity | Moderate; depends on solvent polarity | High; tunable by pressure and temperature |
| Environmental Impact | Higher; solvent disposal issues | Low; eco-friendly and recyclable CO2 |
| Extraction Efficiency | Good, but slower and less specific | High; rapid and precise extraction |
| Process Temperature | Variable; often uses heat which may degrade compounds | Moderate (31-80degC); preserves thermolabile substances |
| Cost | Lower initial investment; higher operational cost (solvent use) | Higher initial investment; lower operating cost |
| Application in Pharmaceuticals | Common for active ingredient isolation but limited by solvent residues | Preferred for pure, residue-free extracts and sensitive compounds |
Introduction to Extraction Techniques
Solvent extraction uses liquid solvents like hexane or ethanol to dissolve target compounds from solid or liquid matrices, offering simplicity and cost-effectiveness but often requiring extensive solvent removal. Supercritical fluid extraction employs supercritical CO2, combining gas-like diffusion with liquid-like solvating power, resulting in efficient, selective extraction with minimal solvent residues. Your choice depends on factors such as compound polarity, environmental impact, and extraction efficiency desired for the application.
Overview of Solvent Extraction
Solvent extraction is a widely used separation process where a specific solvent selectively dissolves a desired compound from a mixture, relying on differences in solubility and chemical affinity. This technique is commonly applied in industries such as pharmaceuticals, food processing, and petrochemicals due to its efficiency in isolating bioactive compounds, essential oils, and metals. Compared to supercritical fluid extraction, solvent extraction often involves longer processing times and potential solvent residues, but it remains favored for its simplicity and cost-effectiveness in large-scale operations.
Fundamentals of Supercritical Fluid Extraction
Supercritical Fluid Extraction (SFE) utilizes fluids above their critical temperature and pressure, where they exhibit unique properties between liquids and gases, allowing efficient solvation and rapid mass transfer. CO2 is the most common solvent in SFE due to its low toxicity, moderate critical conditions (31.1degC, 73.8 bar), and easy removal post-extraction, making it ideal for extracting thermally sensitive compounds. Your choice between Solvent Extraction and SFE depends on factors like solvent residues, extraction speed, and selectivity, with SFE offering greener and more controlled extraction processes.
Comparative Mechanisms of Action
Solvent extraction relies on the solubility of target compounds in liquid solvents to separate components based on polarity differences, often requiring heat to enhance diffusion and solubility. Supercritical fluid extraction utilizes supercritical CO2, which combines gas-like diffusion properties with liquid-like solvating power, enabling selective and efficient extraction at moderate temperatures and pressures. The tunable density and solvating strength of supercritical fluids provide superior selectivity and faster mass transfer compared to conventional solvent extraction methods.
Efficiency and Selectivity Analysis
Solvent extraction offers broad applicability with variable efficiency depending on solvent polarity and target compound solubility, but it often lacks selectivity, leading to co-extraction of impurities. Supercritical fluid extraction (SFE), particularly with supercritical CO2, delivers higher selectivity due to tunable solvating power by adjusting temperature and pressure, enhancing the extraction of specific bioactive compounds with minimal solvent residues. Your choice between these methods should consider the trade-offs in extraction efficiency, selectivity for desired components, and environmental impact of solvents used.
Safety and Environmental Impact
Solvent extraction often involves the use of volatile organic compounds (VOCs) that pose health risks, flammability, and environmental pollution due to solvent disposal challenges. Supercritical fluid extraction, particularly using CO2, offers a safer alternative by eliminating toxic solvents, reducing flammability hazards, and minimizing environmental impact through recyclable and non-toxic extraction agents. The sustainable nature of supercritical fluid extraction aligns with green chemistry principles, making it preferable for industries prioritizing safety and environmental responsibility.
Cost Considerations and Scalability
Solvent extraction typically involves lower initial investment costs but incurs higher operational expenses due to solvent purchase, handling, and disposal, making it less cost-efficient at larger scales. Supercritical fluid extraction demands significant upfront capital for high-pressure equipment and specialized infrastructure but offers lower recurring costs through solvent reuse and reduced waste management expenses. Scalability favors supercritical fluid extraction for industrial applications due to its continuous processing capability and environmentally friendly profile, whereas solvent extraction remains more suitable for small to medium batch sizes due to simpler equipment and process flexibility.
Applications in Industry
Solvent extraction is widely used in the pharmaceutical, food, and petrochemical industries for separating bioactive compounds, essential oils, and metal ions due to its cost-effectiveness and scalability. Supercritical fluid extraction, particularly using CO2, is favored in the food and beverage industry for decaffeination and flavor extraction, as well as in pharmaceuticals for producing high-purity extracts without solvent residues. Both methods serve critical roles in environmental remediation and waste valorization, with supercritical fluid extraction offering greener alternatives to traditional solvent use.
Advantages and Limitations
Solvent extraction offers versatility and cost-effectiveness for extracting a wide range of compounds but often involves toxic solvents and longer processing times. Supercritical fluid extraction provides higher selectivity, faster extraction rates, and environmentally friendly CO2 usage but requires expensive equipment and is less effective for polar compounds. Understanding these advantages and limitations helps you choose the most suitable extraction method for your specific application.
Future Trends in Extraction Technologies
Future trends in extraction technologies emphasize sustainability and efficiency, with supercritical fluid extraction (SFE) gaining prominence due to its reduced environmental impact and ability to selectively extract bioactive compounds without toxic solvents. Solvent extraction remains widely used for its simplicity and cost-effectiveness, but innovations focus on greener solvents and process intensification to minimize solvent consumption and enhance recovery rates. Integration of advanced techniques like microwave-assisted extraction with supercritical fluids is expected to improve extraction yield and reduce processing time, driving the evolution of next-generation extraction methods.
Solvent extraction vs Supercritical fluid extraction Infographic
 libmatt.com
libmatt.com