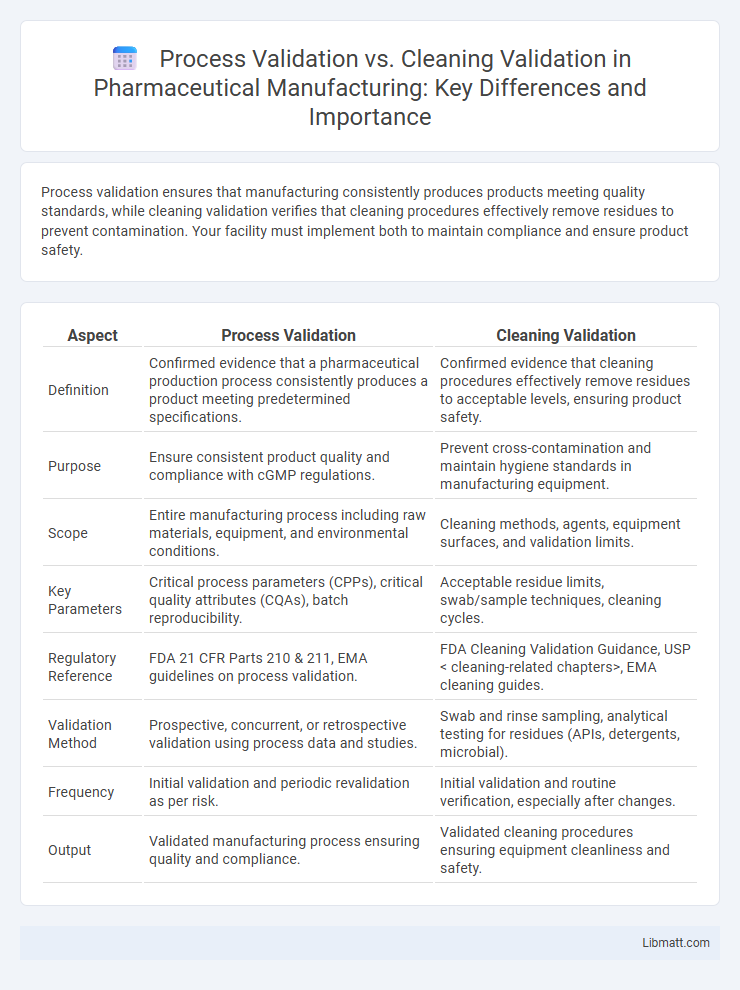
Process validation ensures that manufacturing consistently produces products meeting quality standards, while cleaning validation verifies that cleaning procedures effectively remove residues to prevent contamination. Your facility must implement both to maintain compliance and ensure product safety.
Table of Comparison
| Aspect | Process Validation | Cleaning Validation |
|---|---|---|
| Definition | Confirmed evidence that a pharmaceutical production process consistently produces a product meeting predetermined specifications. | Confirmed evidence that cleaning procedures effectively remove residues to acceptable levels, ensuring product safety. |
| Purpose | Ensure consistent product quality and compliance with cGMP regulations. | Prevent cross-contamination and maintain hygiene standards in manufacturing equipment. |
| Scope | Entire manufacturing process including raw materials, equipment, and environmental conditions. | Cleaning methods, agents, equipment surfaces, and validation limits. |
| Key Parameters | Critical process parameters (CPPs), critical quality attributes (CQAs), batch reproducibility. | Acceptable residue limits, swab/sample techniques, cleaning cycles. |
| Regulatory Reference | FDA 21 CFR Parts 210 & 211, EMA guidelines on process validation. | FDA Cleaning Validation Guidance, USP < cleaning-related chapters>, EMA cleaning guides. |
| Validation Method | Prospective, concurrent, or retrospective validation using process data and studies. | Swab and rinse sampling, analytical testing for residues (APIs, detergents, microbial). |
| Frequency | Initial validation and periodic revalidation as per risk. | Initial validation and routine verification, especially after changes. |
| Output | Validated manufacturing process ensuring quality and compliance. | Validated cleaning procedures ensuring equipment cleanliness and safety. |
Introduction to Process Validation and Cleaning Validation
Process validation ensures that manufacturing procedures consistently produce products meeting predetermined quality criteria, emphasizing equipment, materials, and process parameters. Cleaning validation verifies that cleaning methods effectively remove residues, contaminants, and microbial impurities to prevent cross-contamination and ensure product safety. Your quality management system must integrate both validations to maintain compliance with regulatory standards and guarantee product integrity.
Definitions: Process Validation vs Cleaning Validation
Process validation ensures manufacturing processes consistently produce products meeting predetermined quality standards, focusing on critical parameters and overall performance. Cleaning validation verifies that cleaning procedures effectively remove residues, contaminants, and microbial forms from equipment to prevent cross-contamination and ensure product safety. Both validations are essential in pharmaceutical and biotech industries, adhering to regulatory guidelines such as FDA and EMA for maintaining quality compliance.
Regulatory Guidelines for Validation Practices
Regulatory guidelines for validation practices emphasize distinct requirements for process validation and cleaning validation to ensure product quality and patient safety. Process validation, governed by FDA's 21 CFR Part 211 and ICH Q7, focuses on confirming manufacturing processes consistently produce a product meeting predetermined specifications. Cleaning validation, detailed in FDA guidance and EMA's GMP Annex 15, ensures residue limits are controlled to prevent cross-contamination and maintains the integrity of subsequent batches, guiding your compliance strategies effectively.
Key Objectives of Process Validation
Process validation aims to ensure manufacturing processes consistently produce products meeting predetermined quality criteria, focusing on reproducibility and compliance with regulatory standards such as FDA and EMA guidelines. Key objectives include verifying process parameters, equipment functionality, and control mechanisms to minimize variability and ensure product safety and efficacy. This validation supports risk management and continuous improvement by identifying critical control points throughout production.
Key Objectives of Cleaning Validation
The key objectives of cleaning validation focus on ensuring the removal of residues from manufacturing equipment to prevent cross-contamination and ensure product safety. It aims to verify that cleaning procedures are consistently effective in eliminating harmful contaminants, including active pharmaceutical ingredients (APIs), cleaning agents, and microbial residues. Cleaning validation supports compliance with regulatory standards by demonstrating that cleaning processes maintain product quality and protect patient health.
Steps Involved in Process Validation
Process validation involves comprehensive steps including process design, process qualification, and ongoing process verification to ensure consistent product quality. It starts with defining critical process parameters and quality attributes, followed by executing validation batches under controlled conditions to demonstrate reproducibility. Data analysis and documentation confirm the process operates within predefined limits to meet regulatory standards, distinguishing it from cleaning validation focused on residue removal.
Steps Involved in Cleaning Validation
Cleaning validation involves critical steps such as establishing a cleaning procedure, selecting appropriate analytical methods for residue detection, and setting acceptance limits based on toxicological evaluation. Swab sampling, rinse sampling, and visual inspection are commonly employed techniques to verify the cleanliness of equipment surfaces. Documentation, including validation protocols, sampling plans, and final reports, ensures compliance with regulatory standards like FDA and EMA guidelines.
Major Differences Between Process and Cleaning Validation
Process validation ensures that manufacturing procedures consistently produce products meeting predefined quality standards, focusing on parameters like temperature, pressure, and time during production. Cleaning validation verifies that cleaning methods effectively remove residues, contaminants, or microorganisms to prevent cross-contamination and ensure equipment is safe for subsequent use. Your understanding of these major differences helps maintain both product safety and regulatory compliance in pharmaceutical manufacturing.
Common Challenges in Process and Cleaning Validation
Common challenges in process and cleaning validation include ensuring consistent reproducibility and compliance with regulatory standards such as FDA and EMA guidelines. Variability in raw materials, equipment performance, and environmental conditions can complicate establishing validated states. You must implement robust risk assessment strategies and continuous monitoring to maintain validation integrity and prevent cross-contamination or process deviations.
Best Practices for Effective Validation Strategies
Process validation and cleaning validation require distinct best practices to ensure product quality and regulatory compliance. Process validation focuses on demonstrating that manufacturing steps consistently produce a product meeting predefined criteria, emphasizing critical process parameters and control strategies. Cleaning validation centers on verifying that residual contaminants are effectively removed, highlighting sampling methods and limits to protect Your product from cross-contamination risks.
Process validation vs cleaning validation Infographic
 libmatt.com
libmatt.com