Cleaning validation ensures that residues from previous batches, cleaning agents, and microbial contaminants are effectively removed from equipment, guaranteeing product safety and quality. Sanitization validation focuses on reducing microbial load to acceptable levels, which is crucial for preventing contamination during the manufacturing process.
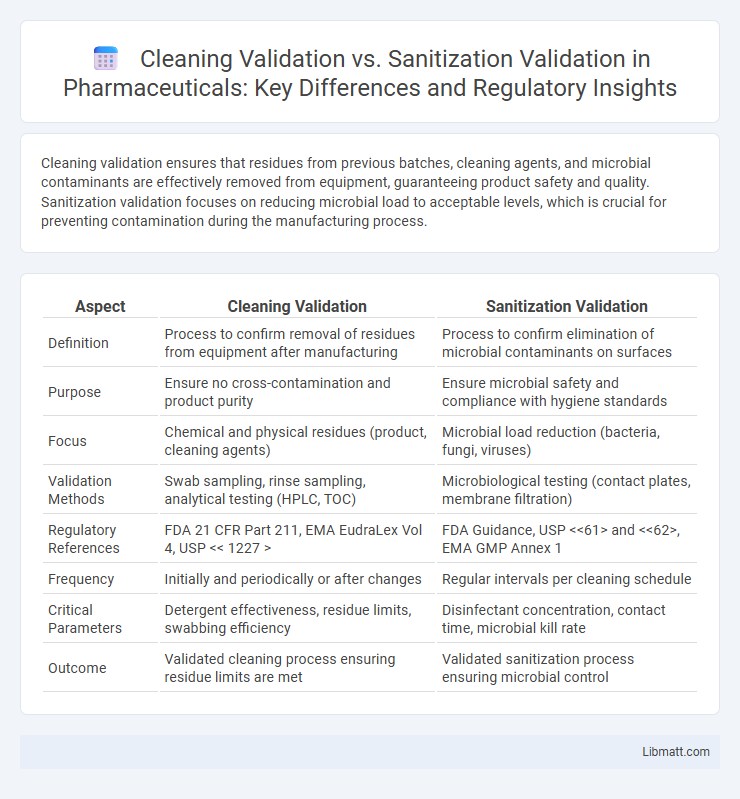
Table of Comparison
| Aspect | Cleaning Validation | Sanitization Validation |
|---|---|---|
| Definition | Process to confirm removal of residues from equipment after manufacturing | Process to confirm elimination of microbial contaminants on surfaces |
| Purpose | Ensure no cross-contamination and product purity | Ensure microbial safety and compliance with hygiene standards |
| Focus | Chemical and physical residues (product, cleaning agents) | Microbial load reduction (bacteria, fungi, viruses) |
| Validation Methods | Swab sampling, rinse sampling, analytical testing (HPLC, TOC) | Microbiological testing (contact plates, membrane filtration) |
| Regulatory References | FDA 21 CFR Part 211, EMA EudraLex Vol 4, USP << 1227 > | FDA Guidance, USP <<61> and <<62>, EMA GMP Annex 1 |
| Frequency | Initially and periodically or after changes | Regular intervals per cleaning schedule |
| Critical Parameters | Detergent effectiveness, residue limits, swabbing efficiency | Disinfectant concentration, contact time, microbial kill rate |
| Outcome | Validated cleaning process ensuring residue limits are met | Validated sanitization process ensuring microbial control |
Introduction to Cleaning and Sanitization Validation
Cleaning validation ensures that pharmaceutical equipment is free from residual contaminants, verifying that cleaning processes consistently remove active ingredients, excipients, and microbial residues. Sanitization validation focuses on reducing microbial contamination to acceptable levels, confirming that sanitizing agents effectively control bioburden on surfaces. Both validations are critical for maintaining product safety, quality, and compliance with regulatory standards such as FDA and EMA guidelines.
Definitions: Cleaning Validation vs. Sanitization Validation
Cleaning validation ensures that manufacturing equipment is free from contaminants, residues, and microbial impurities to acceptable levels after cleaning processes. Sanitization validation focuses on confirming that sanitation procedures effectively reduce pathogenic microorganisms to safe limits on surfaces and equipment. Both validations are critical in pharmaceutical and food industries to maintain product safety and compliance with regulatory standards.
Regulatory Requirements and Guidelines
Cleaning validation ensures that equipment and surfaces are free from residues and contaminants to meet regulatory requirements set by agencies like the FDA and EMA, which mandate documented evidence of effectiveness. Sanitization validation focuses on reducing microbial load to an acceptable level, adhering to guidelines such as USP <1072> and WHO standards for ensuring hygienic manufacturing environments. Your compliance strategy should align these validations with specific regulatory frameworks to maintain product safety and quality.
Key Objectives of Cleaning Validation
Cleaning validation ensures that residues, contaminants, or microbial contaminants are removed to acceptable levels, safeguarding product quality and patient safety. The key objectives focus on demonstrating the effectiveness, reproducibility, and consistency of the cleaning process, confirming that no harmful residues remain on equipment. Your facility must establish validated procedures that comply with regulatory standards to prevent cross-contamination and maintain product integrity.
Key Objectives of Sanitization Validation
Sanitization validation ensures microbial control by verifying that cleaning processes effectively reduce harmful microorganisms on surfaces to an acceptable risk level. It focuses on confirming that sanitizers and cleaning agents consistently achieve the desired bioburden reduction without adversely affecting equipment integrity. Your manufacturing environment benefits from validated sanitization to maintain product safety and regulatory compliance.
Methods and Approaches for Validation
Cleaning validation employs swab sampling, rinse sampling, and analytical methods such as high-performance liquid chromatography (HPLC) or total organic carbon (TOC) analysis to quantitatively assess residual contaminants on manufacturing equipment. Sanitization validation primarily focuses on microbial reduction verification using bioburden testing, microbial enumeration, and disinfectant efficacy assays to ensure pathogen control on surfaces. Both processes require establishing acceptance criteria and risk-based protocols aligned with regulatory guidelines like FDA and EMA to confirm consistency and effectiveness in contamination control.
Critical Parameters in Cleaning Validation
Critical parameters in cleaning validation include equipment design, residue chemistry, cleaning procedure effectiveness, and detection limits to ensure product safety and compliance. Sanitization validation focuses more on microbial control, requiring parameters like contact time, concentration of sanitizing agents, and surface compatibility. Understanding these distinctions helps you maintain rigorous quality standards and regulatory adherence in pharmaceutical manufacturing.
Critical Parameters in Sanitization Validation
Sanitization validation primarily focuses on critical parameters such as contact time, concentration of sanitizing agents, temperature, and mechanical action to ensure effective microbial reduction on equipment surfaces. These parameters are meticulously controlled to prevent cross-contamination and maintain hygienic conditions in pharmaceutical and food manufacturing environments. Unlike cleaning validation, which emphasizes residue removal, sanitization validation centers on achieving microbial control to uphold product safety and quality.
Common Challenges and Solutions
Cleaning validation and sanitization validation share common challenges such as ensuring consistent removal of contaminants and verifying effectiveness across diverse equipment surfaces. Difficulties include selecting appropriate analytical methods, managing variability in residue types, and maintaining regulatory compliance. Solutions involve implementing robust risk-based approaches, using validated test methods like ATP bioluminescence or HPLC, and establishing thorough documentation and routine requalification protocols.
Industry Best Practices and Compliance Considerations
Cleaning validation ensures that your manufacturing equipment is free from contaminants, residues, and microbial hazards to meet regulatory standards such as FDA and EMA guidelines. Sanitization validation focuses on verifying the effectiveness of sanitation processes in reducing microbial load to acceptable levels, critical in food, pharmaceutical, and biotech industries. Adhering to industry best practices involves rigorous risk assessments, documented protocols, and periodic revalidation to maintain compliance and ensure product safety and quality.
Cleaning validation vs Sanitization validation Infographic
 libmatt.com
libmatt.com