In clinical trials, a double-blind design ensures that neither the participants nor the researchers know who receives the treatment or placebo, minimizing bias and enhancing the reliability of results. A single-blind study keeps participants unaware of their group assignment while researchers remain informed, which may reduce participant bias but allows for potential observer influence.
Table of Comparison
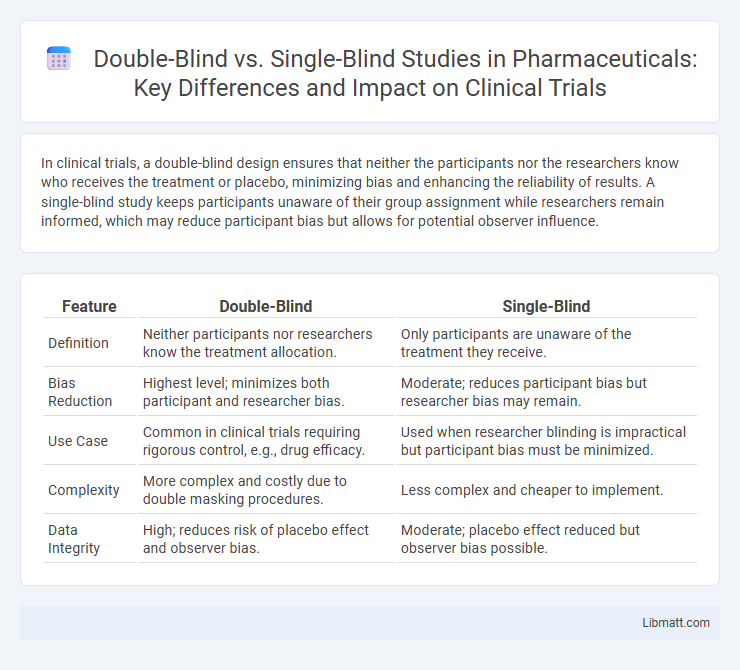
| Feature | Double-Blind | Single-Blind |
|---|---|---|
| Definition | Neither participants nor researchers know the treatment allocation. | Only participants are unaware of the treatment they receive. |
| Bias Reduction | Highest level; minimizes both participant and researcher bias. | Moderate; reduces participant bias but researcher bias may remain. |
| Use Case | Common in clinical trials requiring rigorous control, e.g., drug efficacy. | Used when researcher blinding is impractical but participant bias must be minimized. |
| Complexity | More complex and costly due to double masking procedures. | Less complex and cheaper to implement. |
| Data Integrity | High; reduces risk of placebo effect and observer bias. | Moderate; placebo effect reduced but observer bias possible. |
Introduction to Blinding in Research
Blinding in research is a crucial methodological technique designed to minimize bias by preventing participants, researchers, or both from knowing specific details of the study. In single-blind studies, only the participants are unaware of the treatment allocation, whereas double-blind studies keep both participants and researchers uninformed to enhance objectivity. Understanding the distinction between double-blind and single-blind methods can help you critically assess the reliability and validity of clinical trial results.
Definition of Single-Blind Studies
Single-blind studies are research designs where participants remain unaware of whether they receive the treatment or placebo, reducing bias in self-reporting and placebo effects. However, researchers conducting the study know the group assignments, which may introduce observer bias. Your understanding of single-blind studies helps in critically evaluating research validity and reliability.
Definition of Double-Blind Studies
Double-blind studies are research designs where neither the participants nor the experimenters know who receives the treatment or placebo, reducing bias and increasing validity. This method ensures that both the subjects' and researchers' expectations do not influence the results, enhancing the reliability of clinical trials. Your understanding of double-blind protocols helps in critically evaluating the credibility of scientific findings.
Key Differences between Single-Blind and Double-Blind
Single-blind studies keep participants unaware of their group assignment to reduce bias, while researchers know the allocation; double-blind studies conceal information from both participants and researchers to minimize bias more effectively. The key difference lies in the level of blinding, impacting the reliability of results and reducing placebo effects. Your choice of study design influences the control of bias and the validity of clinical or experimental outcomes.
Importance of Blinding for Reducing Bias
Blinding is crucial in clinical trials to reduce bias and ensure the validity of results. In double-blind studies, neither participants nor researchers know who receives the treatment or placebo, minimizing both performance and detection bias. Single-blind trials keep subjects unaware but may still allow researcher bias, making double-blind methods more reliable for unbiased outcomes.
Advantages of Double-Blind Designs
Double-blind designs eliminate bias by ensuring that neither participants nor researchers know who receives the treatment, enhancing the study's objectivity. This approach increases the reliability and validity of results by minimizing placebo effects and observer bias. Choosing a double-blind design can strengthen your research conclusions and improve the credibility of your findings.
Limitations of Single-Blind Studies
Single-blind studies, where only participants are unaware of the treatment allocation, may introduce researcher bias as investigators are informed and can unintentionally influence outcomes. This limitation reduces the reliability of subjective measures and increases the risk of placebo effects affecting results. For your study's integrity, consider that double-blind designs more effectively minimize bias by keeping both researchers and participants unaware of group assignments.
Real-World Examples: Single-Blind vs Double-Blind
In clinical trials for new medications, single-blind studies often involve patients unaware of their treatment group, while researchers know the assignments to monitor results closely. Double-blind studies, used in vaccine testing, keep both participants and researchers unaware of group allocations to eliminate bias and enhance objectivity. For example, a double-blind trial by Pfizer for the COVID-19 vaccine ensured unbiased efficacy results, whereas a single-blind trial for pain medications allowed researchers to adjust dosages based on observed side effects.
Choosing the Right Blinding Method
Selecting the appropriate blinding method hinges on the study's objectives and potential bias sources, where double-blind designs conceal treatment allocation from both participants and researchers to minimize placebo effects and observer bias. Single-blind trials, keeping only participants unaware, reduce bias primarily from participant expectations but may not prevent investigator influence on data collection or interpretation. Evaluating factors like intervention type, ethical considerations, and logistical feasibility guides the choice between single-blind and double-blind approaches to ensure valid and reliable clinical trial outcomes.
Conclusion: Impact of Blinding on Research Validity
Blinding significantly enhances research validity by minimizing bias and ensuring objective data collection; double-blind studies, where both participants and researchers are unaware of group assignments, offer higher protection against placebo effects and observer bias compared to single-blind designs. Single-blind studies, though effective in reducing participant bias, remain susceptible to researcher influence, potentially compromising outcome integrity. Employing double-blind protocols maximizes the credibility of results, particularly in clinical trials and experimental research, by strengthening internal validity and reproducibility.
Double-blind vs single-blind Infographic
 libmatt.com
libmatt.com