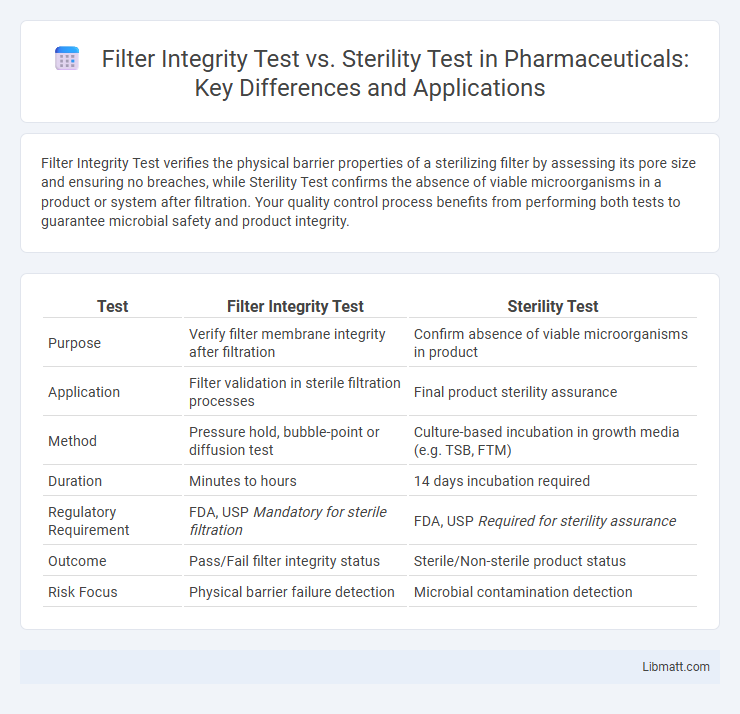
Filter Integrity Test verifies the physical barrier properties of a sterilizing filter by assessing its pore size and ensuring no breaches, while Sterility Test confirms the absence of viable microorganisms in a product or system after filtration. Your quality control process benefits from performing both tests to guarantee microbial safety and product integrity.
Table of Comparison
| Test | Filter Integrity Test | Sterility Test |
|---|---|---|
| Purpose | Verify filter membrane integrity after filtration | Confirm absence of viable microorganisms in product |
| Application | Filter validation in sterile filtration processes | Final product sterility assurance |
| Method | Pressure hold, bubble-point or diffusion test | Culture-based incubation in growth media (e.g. TSB, FTM) |
| Duration | Minutes to hours | 14 days incubation required |
| Regulatory Requirement | FDA, USP Mandatory for sterile filtration | FDA, USP Required for sterility assurance |
| Outcome | Pass/Fail filter integrity status | Sterile/Non-sterile product status |
| Risk Focus | Physical barrier failure detection | Microbial contamination detection |
Introduction to Filter Integrity Test and Sterility Test
Filter Integrity Test evaluates the physical condition and performance of sterilizing-grade filters used in pharmaceutical production, ensuring no breaches or membrane damage before product filtration. Sterility Test confirms the absence of viable microorganisms in the final pharmaceutical product, verifying that it is free from microbial contamination after filtration and processing. Both tests are critical quality control measures in aseptic manufacturing, with Filter Integrity Test preventing contamination risk upfront, while Sterility Test provides final assurance of product safety.
Definitions and Core Principles
Filter Integrity Test assesses the physical and functional condition of sterilizing filters by measuring parameters like bubble point or diffusion flow to ensure they are intact and capable of removing microorganisms. Sterility Test evaluates the absence of viable microorganisms in a product by incubating samples under specific conditions to confirm the product's microbial safety. Your quality control relies on Filter Integrity Testing for real-time assurance of filter performance, while Sterility Testing provides definitive validation of microbial sterility.
Purpose and Application in Pharmaceutical Processes
Filter Integrity Test ensures the physical effectiveness of sterilizing filters by detecting defects or breaches, serving as a critical quality control step before and after sterile filtration in pharmaceutical manufacturing. Sterility Test verifies the absence of viable microorganisms in final pharmaceutical products, confirming product safety and compliance with regulatory standards. Both tests are essential for aseptic processing, with Filter Integrity Test preventing contamination during filtration and Sterility Test validating the sterility of the end product.
Methodologies: How Each Test Is Performed
The Filter Integrity Test evaluates filter performance by measuring parameters such as bubble point pressure or diffusive airflow to ensure filter pores are intact and functional. The Sterility Test involves incubating a sample of the filtered product in culture media for an extended period, typically 14 days, to detect microbial contamination. Your choice depends on whether you need to verify the physical integrity of the filter or confirm the absence of viable microorganisms in the final product.
Key Differences Between Filter Integrity and Sterility Tests
Filter Integrity Test evaluates the physical performance and pore size retention of sterilizing-grade filters, ensuring no damage or breach before use. Sterility Test confirms the absence of viable microorganisms in a product after filtration, validating the effectiveness of the sterilization process. Filter Integrity is a nondestructive, in-line assessment typically performed pre- or post-filtration, while Sterility Test is a biological assay requiring incubation over several days to detect microbial contamination.
Regulatory Requirements and Industry Standards
Filter Integrity Test ensures membrane filters meet regulatory requirements by verifying pore size and integrity before sterilization, aligning with USP <1207> and FDA guidelines. Sterility Test complies with industry standards such as USP <71> and EMA regulations by confirming the absence of viable microorganisms in the final product. Your compliance strategy should integrate both tests to meet rigorous quality assurance and regulatory expectations in pharmaceutical manufacturing.
Timing: When to Conduct Each Test
Filter integrity tests must be conducted immediately after filtration to verify the filter's ability to retain microorganisms, ensuring the process is intact before sterility testing. Sterility tests are performed after product manufacturing and filtration, typically following a suitable incubation period, to confirm the absence of viable contaminants. Proper timing ensures reliable validation of both filtration efficacy and final product sterility.
Data Interpretation and Result Significance
Filter Integrity Test evaluates the physical performance of sterilizing filters by measuring parameters like bubble point and diffusion flow rates to ensure effective microbial retention before use. Sterility Test verifies the absence of viable microorganisms in the final product by incubating samples under specific conditions and analyzing microbial growth over a defined period. Understanding the distinct data from both tests is crucial: Filter Integrity results confirm filter functionality, while Sterility Test outcomes confirm product safety, enabling you to ensure compliance with sterility assurance levels in pharmaceutical manufacturing.
Common Challenges and Troubleshooting
Filter integrity test and sterility test both face challenges such as detecting filter breaches and ensuring no microbial contamination, respectively, with common issues including false positives or negatives due to improper test conditions or equipment malfunction. Troubleshooting often requires calibration of instruments, verification of test parameters like pressure and flow rates for filter integrity, and stringent environmental controls and aseptic techniques for sterility testing. Maintaining rigorous validation protocols and regular staff training enhances reliability in detecting filter failures and sterility assurance.
Choosing the Right Test for Your Process
Selecting the right test for your process depends on critical factors such as regulatory requirements, product type, and risk level. Filter Integrity Tests verify membrane integrity and are ideal for routine in-process checks, ensuring your filtration system functions correctly without compromising sterility. Sterility Tests, while more time-consuming, are essential for final product validation to confirm the absence of viable microorganisms, delivering definitive assurance of product safety.
Filter Integrity Test vs Sterility Test Infographic
 libmatt.com
libmatt.com