The Target Product Profile defines the desired characteristics and performance goals for a product throughout its development, guiding formulation and clinical strategies. Your QTPP (Quality Target Product Profile) focuses specifically on the quality attributes that must be met to ensure safety, efficacy, and regulatory compliance during manufacturing and lifecycle management.
Table of Comparison
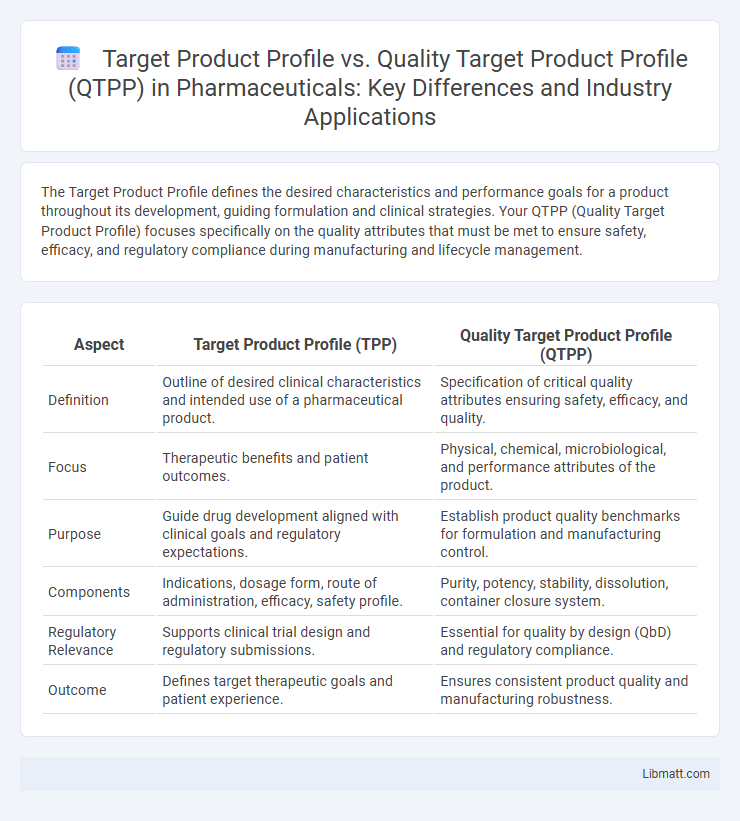
| Aspect | Target Product Profile (TPP) | Quality Target Product Profile (QTPP) |
|---|---|---|
| Definition | Outline of desired clinical characteristics and intended use of a pharmaceutical product. | Specification of critical quality attributes ensuring safety, efficacy, and quality. |
| Focus | Therapeutic benefits and patient outcomes. | Physical, chemical, microbiological, and performance attributes of the product. |
| Purpose | Guide drug development aligned with clinical goals and regulatory expectations. | Establish product quality benchmarks for formulation and manufacturing control. |
| Components | Indications, dosage form, route of administration, efficacy, safety profile. | Purity, potency, stability, dissolution, container closure system. |
| Regulatory Relevance | Supports clinical trial design and regulatory submissions. | Essential for quality by design (QbD) and regulatory compliance. |
| Outcome | Defines target therapeutic goals and patient experience. | Ensures consistent product quality and manufacturing robustness. |
Understanding Target Product Profile (TPP)
Target Product Profile (TPP) defines a drug's desired characteristics, including dosage form, route of administration, and therapeutic indications, serving as a strategic development guide. Quality Target Product Profile (QTPP) specifically outlines the quality attributes necessary to ensure safety and efficacy, guiding formulation and manufacturing processes. Understanding TPP enables alignment of product development goals with regulatory expectations and market needs.
Defining Quality Target Product Profile (QTPP)
Quality Target Product Profile (QTPP) defines the essential quality characteristics a pharmaceutical product must possess to ensure desired safety, efficacy, and performance. QTPP includes parameters such as dosage form, route of administration, strength, and stability criteria aligned with regulatory expectations. Establishing a precise QTPP guides formulation development and risk assessment during drug product lifecycle management.
Key Objectives of TPP and QTPP
Target Product Profile (TPP) defines the desired characteristics and key attributes of a drug product to guide development and regulatory approval. Quality Target Product Profile (QTPP) specifies measurable quality attributes to ensure the final pharmaceutical product meets safety, efficacy, and performance standards. Understanding Your product's TPP and QTPP helps align development efforts with clinical goals and regulatory requirements to optimize therapeutic outcomes.
Differences Between TPP and QTPP
The Target Product Profile (TPP) outlines the intended use, safety, efficacy, and labeling goals for a drug during development, serving as a strategic planning tool. The Quality Target Product Profile (QTPP) specifically defines the desired quality characteristics related to the drug product's physical, chemical, microbiological, and performance attributes necessary to ensure safety and efficacy. While TPP focuses broadly on clinical and commercial objectives, QTPP concentrates on measurable quality parameters critical for regulatory approval and consistent manufacturing.
Regulatory Significance of TPP vs QTPP
The Target Product Profile (TPP) serves as a strategic regulatory tool that outlines the desired characteristics and intended use of a drug product, guiding regulatory submissions and labeling decisions. In contrast, the Quality Target Product Profile (QTPP) specifically focuses on quality attributes impacting manufacturing and product performance, aligning with regulatory expectations for product consistency and safety. Your understanding of both profiles enhances regulatory strategy by ensuring compliance with agency requirements and facilitating product approval processes.
Role of TPP in Drug Development
The Target Product Profile (TPP) outlines the desired characteristics and intended use of a drug, serving as a strategic roadmap throughout drug development. It informs critical decisions on formulation, clinical trial design, and regulatory strategies by defining efficacy, safety, dosage, and route of administration benchmarks. Aligning the TPP with the Quality Target Product Profile (QTPP) ensures that quality attributes meet clinical and regulatory requirements, facilitating successful product approval and market access.
QTPP in Pharmaceutical Quality by Design (QbD)
Quality Target Product Profile (QTPP) serves as a foundational element in Pharmaceutical Quality by Design (QbD), defining the desired quality characteristics essential for ensuring product safety and efficacy. QTPP guides the systematic development process by aligning critical quality attributes (CQAs) with therapeutic objectives and regulatory requirements. Effective implementation of QTPP enables risk-based decision-making and streamlines control strategies to consistently achieve quality in pharmaceutical products.
Overlap and Interconnection: TPP and QTPP
The Target Product Profile (TPP) outlines the intended clinical attributes and patient benefits of a drug, while the Quality Target Product Profile (QTPP) defines the desired quality characteristics ensuring consistent performance. Both frameworks overlap in establishing product expectations that guide development, with QTPP translating the clinical goals of the TPP into precise quality attributes for manufacturing. This interconnection facilitates alignment between therapeutic objectives and quality control, enabling regulatory compliance and optimized drug efficacy.
Best Practices for Developing TPP and QTPP
Developing a Target Product Profile (TPP) and Quality Target Product Profile (QTPP) requires aligning product attributes with regulatory requirements and patient needs, emphasizing critical quality attributes (CQAs) and clinical endpoints. Best practices include incorporating risk management strategies, cross-functional collaboration among regulatory, clinical, and manufacturing teams, and continuous revision based on emerging data to ensure comprehensive product specifications. Utilizing robust analytical tools and integrating feedback from stability, efficacy, and safety studies optimize the definition and control of quality parameters throughout the product lifecycle.
Strategic Integration of TPP and QTPP in Product Lifecycle
Target Product Profile (TPP) and Quality Target Product Profile (QTPP) serve as foundational tools for strategic product development and regulatory alignment throughout the product lifecycle. Integrating TPP with QTPP ensures that clinical, safety, efficacy, and quality attributes are coherently aligned from early development phases to commercialization, facilitating risk mitigation and compliance. This synchronization streamlines decision-making, optimizes resource allocation, and supports consistent product quality and performance in the market.
Target product profile vs QTPP (Quality Target Product Profile) Infographic
 libmatt.com
libmatt.com