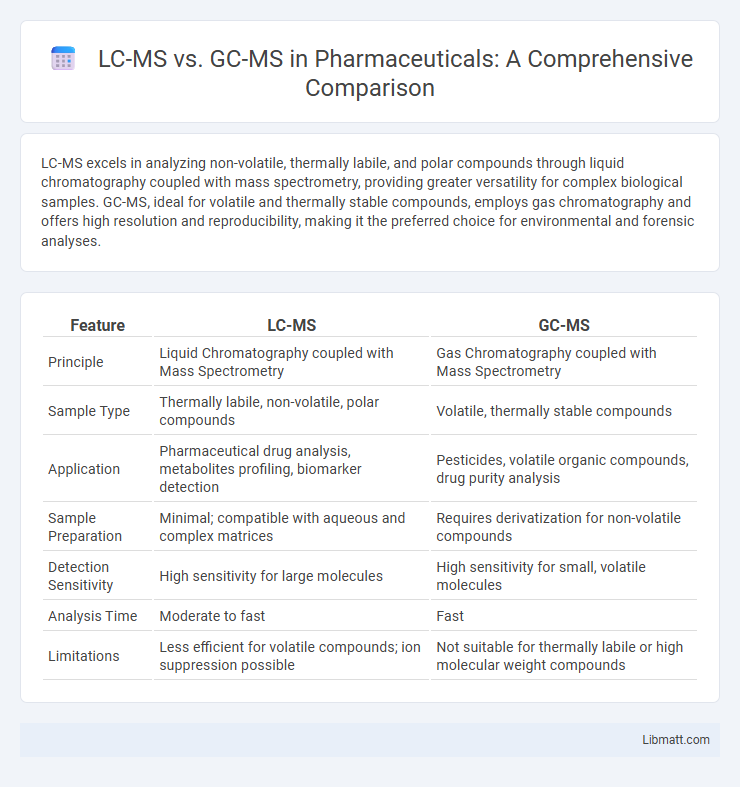
LC-MS excels in analyzing non-volatile, thermally labile, and polar compounds through liquid chromatography coupled with mass spectrometry, providing greater versatility for complex biological samples. GC-MS, ideal for volatile and thermally stable compounds, employs gas chromatography and offers high resolution and reproducibility, making it the preferred choice for environmental and forensic analyses.
Table of Comparison
| Feature | LC-MS | GC-MS |
|---|---|---|
| Principle | Liquid Chromatography coupled with Mass Spectrometry | Gas Chromatography coupled with Mass Spectrometry |
| Sample Type | Thermally labile, non-volatile, polar compounds | Volatile, thermally stable compounds |
| Application | Pharmaceutical drug analysis, metabolites profiling, biomarker detection | Pesticides, volatile organic compounds, drug purity analysis |
| Sample Preparation | Minimal; compatible with aqueous and complex matrices | Requires derivatization for non-volatile compounds |
| Detection Sensitivity | High sensitivity for large molecules | High sensitivity for small, volatile molecules |
| Analysis Time | Moderate to fast | Fast |
| Limitations | Less efficient for volatile compounds; ion suppression possible | Not suitable for thermally labile or high molecular weight compounds |
Introduction to LC-MS and GC-MS
LC-MS (Liquid Chromatography-Mass Spectrometry) combines liquid chromatography's separation capabilities with mass spectrometry's analyte identification, making it ideal for analyzing complex, non-volatile, and thermally labile compounds. GC-MS (Gas Chromatography-Mass Spectrometry) integrates gas chromatography for separating volatile and semi-volatile compounds with mass spectrometry for precise detection and quantification. Your choice between LC-MS and GC-MS depends on the chemical properties of the analyte and the specific analytical requirements.
Fundamental Principles of LC-MS
Liquid Chromatography-Mass Spectrometry (LC-MS) combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry, allowing precise identification and quantification of compounds in complex mixtures. LC-MS relies on the differential partitioning of analytes between a liquid mobile phase and a stationary phase, followed by ionization and mass detection, making it ideal for polar, thermally labile, and high-molecular-weight compounds. Your analysis benefits from LC-MS when targeting molecules that are difficult to vaporize or degrade at high temperatures, setting it apart from Gas Chromatography-Mass Spectrometry (GC-MS).
Fundamental Principles of GC-MS
Gas Chromatography-Mass Spectrometry (GC-MS) combines gas chromatography's ability to separate volatile compounds with mass spectrometry's capacity to identify molecules based on mass-to-charge ratios. In GC, samples are vaporized and carried by an inert gas through a column, where components separate according to their boiling points and interactions with the stationary phase. The separated compounds then enter the mass spectrometer, where they are ionized, fragmented, and detected, enabling precise qualitative and quantitative analysis of complex mixtures.
Key Differences Between LC-MS and GC-MS
LC-MS (Liquid Chromatography-Mass Spectrometry) utilizes liquid chromatography for separating compounds, making it suitable for analyzing thermally labile and non-volatile molecules, while GC-MS (Gas Chromatography-Mass Spectrometry) employs gas chromatography, ideal for volatile and thermally stable substances. LC-MS offers a broader range of analytes, such as proteins, peptides, and polar compounds, whereas GC-MS excels in analyzing small organic molecules and environmental pollutants. The sensitivity and resolution of LC-MS are often favored for complex biological samples, while GC-MS provides high separation efficiency and reproducible results for volatile organic compounds.
Sample Preparation Requirements
LC-MS requires minimal sample preparation since it can analyze a wide range of polar and non-volatile compounds directly in liquid form. GC-MS demands more extensive preparation, including derivatization, to make samples volatile and thermally stable for gas-phase analysis. Your choice depends on the chemical nature of the analytes and the complexity of the sample matrix.
Applications in Various Industries
LC-MS excels in pharmaceutical and biomedical industries for analyzing complex biomolecules like proteins and metabolites due to its ability to handle non-volatile and thermally labile compounds. GC-MS is widely used in environmental monitoring and forensic science for detecting volatile organic compounds and pesticides with high sensitivity and robust chromatographic separation. Both techniques are integral in food safety testing, enabling detailed profiling of contaminants, additives, and trace residues to ensure regulatory compliance.
Sensitivity and Selectivity Comparison
LC-MS offers higher sensitivity for polar, thermally labile compounds due to its ability to analyze a broad range of molecular weights without derivatization. GC-MS provides superior selectivity for volatile and semi-volatile organic compounds with well-established spectral libraries enabling precise compound identification. The choice between LC-MS and GC-MS depends on the chemical nature of analytes and the required detection limits in complex matrices.
Advantages of LC-MS and GC-MS
LC-MS offers superior analysis of thermally labile and polar compounds due to its soft ionization techniques and minimal sample preparation requirements, enabling high sensitivity and specificity in complex biological matrices. GC-MS excels in separating volatile and semi-volatile organic compounds with high resolution and reproducibility, making it ideal for environmental monitoring, forensic analysis, and petrochemical studies. Both techniques provide robust qualitative and quantitative data, but LC-MS is preferred for large biomolecules while GC-MS is favored for small, volatile analytes.
Limitations and Challenges
LC-MS faces challenges with ion suppression and complex sample matrices that can affect sensitivity and quantitation accuracy, limiting its effectiveness for certain compounds. GC-MS requires analytes to be volatile and thermally stable, restricting its use to smaller, less polar molecules and often necessitating extensive sample preparation. Your choice between LC-MS and GC-MS depends on the chemical nature of the analyte and the specific analytical demands of your study.
Choosing the Right Technique for Your Analysis
LC-MS excels in analyzing large, non-volatile, and thermally labile compounds, making it ideal for pharmaceuticals and biomolecules. GC-MS provides superior separation and sensitivity for volatile and semi-volatile organic compounds, often used in environmental and forensic studies. Your choice depends on the chemical nature of the sample, desired sensitivity, and analytical goals to achieve accurate and reliable results.
LC-MS vs GC-MS Infographic
 libmatt.com
libmatt.com