Lyophilization preserves the structural integrity and bioactivity of sensitive materials by freezing and sublimating moisture under low pressure, while spray drying rapidly converts liquids into powders through hot air evaporation, often leading to faster processing times but potential heat-induced degradation. Choosing between lyophilization and spray drying depends on your product's stability requirements, desired particle size, and production efficiency.
Table of Comparison
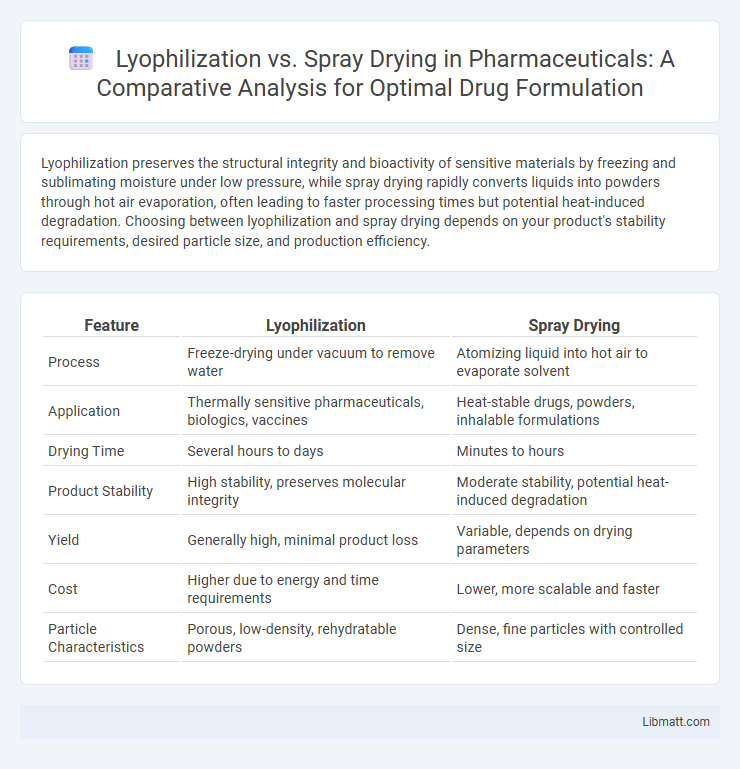
| Feature | Lyophilization | Spray Drying |
|---|---|---|
| Process | Freeze-drying under vacuum to remove water | Atomizing liquid into hot air to evaporate solvent |
| Application | Thermally sensitive pharmaceuticals, biologics, vaccines | Heat-stable drugs, powders, inhalable formulations |
| Drying Time | Several hours to days | Minutes to hours |
| Product Stability | High stability, preserves molecular integrity | Moderate stability, potential heat-induced degradation |
| Yield | Generally high, minimal product loss | Variable, depends on drying parameters |
| Cost | Higher due to energy and time requirements | Lower, more scalable and faster |
| Particle Characteristics | Porous, low-density, rehydratable powders | Dense, fine particles with controlled size |
Introduction to Lyophilization and Spray Drying
Lyophilization, also known as freeze-drying, preserves heat-sensitive substances by freezing the material and then reducing surrounding pressure to allow the frozen water to sublimate. Spray drying involves converting liquid solutions or suspensions into dry powders by rapidly drying with hot gas, making it ideal for heat-stable products. Your choice between lyophilization and spray drying should consider factors such as product sensitivity, drying speed, and desired powder characteristics.
Principles and Processes of Lyophilization
Lyophilization, or freeze-drying, involves freezing the product and reducing surrounding pressure to allow the frozen water to sublimate directly from solid to gas. This process preserves heat-sensitive materials by avoiding liquid phase, maintaining structural and chemical integrity. Your choice of lyophilization ensures enhanced stability and longer shelf life for pharmaceuticals and biologics compared to spray drying.
Principles and Processes of Spray Drying
Spray drying involves atomizing a liquid feed into a hot drying chamber where rapid evaporation transforms droplets into fine powders, preserving the material's integrity. This process relies on the principles of heat and mass transfer, optimizing drying efficiency through parameters like inlet temperature, feed rate, and airflow. Compared to lyophilization, spray drying is faster and more cost-effective for producing powdered pharmaceuticals, food ingredients, and bioproducts with controlled particle size and moisture content.
Key Differences Between Lyophilization and Spray Drying
Lyophilization involves freezing a product and removing moisture through sublimation, preserving heat-sensitive materials with minimal structural damage, while spray drying uses hot air to rapidly evaporate moisture from liquid feed, suitable for large-scale powder production but may affect thermal stability. Lyophilization results in porous, lightweight powders with high reconstitution ability, whereas spray drying produces denser powders with faster solubility but lower preservation of bioactivity. Your choice depends on the product's thermal sensitivity, desired powder characteristics, and cost considerations between both drying methods.
Product Stability and Quality Comparison
Lyophilization offers superior product stability by removing moisture through sublimation, preserving structural integrity and bioactivity in sensitive pharmaceuticals and biologics. Spray drying, while faster and cost-effective, exposes products to higher temperatures, which can degrade heat-sensitive compounds and reduce overall quality. Both methods impact residual moisture content and particle morphology, but lyophilization consistently ensures longer shelf life and enhanced preservation of active ingredients.
Efficiency and Throughput Analysis
Lyophilization offers superior preservation of sensitive compounds but generally requires longer processing times, resulting in lower throughput compared to spray drying. Spray drying excels in high-efficiency production, rapidly converting liquids into powders with continuous operation suitable for large-scale manufacturing. Your choice depends on balancing product stability with operational speed and volume demands.
Application Areas in Pharmaceuticals and Food Industry
Lyophilization is widely used in the pharmaceutical industry for preserving heat-sensitive drugs, vaccines, and biologics by removing moisture through sublimation, ensuring product stability and extended shelf life. Spray drying is extensively applied in the food industry for producing powdered products like milk, coffee, and flavorings, offering rapid drying and improved solubility. Both techniques are pivotal in pharmaceutical formulations and food processing, with lyophilization favoring preservation of complex biomolecules and spray drying enabling large-scale, cost-effective powder production.
Cost Considerations: Lyophilization vs Spray Drying
Lyophilization typically incurs higher costs due to its energy-intensive vacuum and freezing processes, extensive cycle times, and specialized equipment requirements, making it less economical for large-scale production. Spray drying offers a more cost-effective alternative with faster processing times, lower energy consumption, and simpler operation, which reduces labor and operational expenses. Companies must evaluate production volume, product sensitivity, and quality requirements to determine the most cost-efficient drying method between lyophilization and spray drying.
Environmental Impact and Resource Utilization
Lyophilization consumes substantial energy due to prolonged freezing and vacuum processes, leading to higher electricity usage and potential greenhouse gas emissions, whereas spray drying is generally more energy-efficient with quicker drying cycles and lower overall energy consumption. Spray drying often requires less water and generates fewer solvent emissions, reducing environmental impact compared to the extensive resource demands of lyophilization. Your choice between these methods impacts both environmental sustainability and resource utilization, with spray drying typically offering a greener and more resource-efficient option.
Choosing the Right Method for Your Needs
Lyophilization preserves product quality and bioactivity by freezing and sublimating moisture, ideal for heat-sensitive pharmaceuticals and biologics, while spray drying offers rapid drying with lower costs suited for large-scale production of powders and granules. Your choice depends on factors such as product stability, desired particle characteristics, and budget constraints. Consider lyophilization for high-value, temperature-sensitive materials and spray drying for faster processing and scalability.
Lyophilization vs Spray Drying Infographic
 libmatt.com
libmatt.com