Penicillin fermentation typically involves the use of Penicillium mold to produce beta-lactam antibiotics effective against gram-positive bacteria, while cephalosporin fermentation utilizes Acremonium (formerly Cephalosporium) species to generate a broader spectrum of cephalosporin antibiotics with enhanced resistance to beta-lactamase enzymes. Your choice between the two fermentation processes depends on the desired antibiotic properties and target bacterial infections.
Table of Comparison
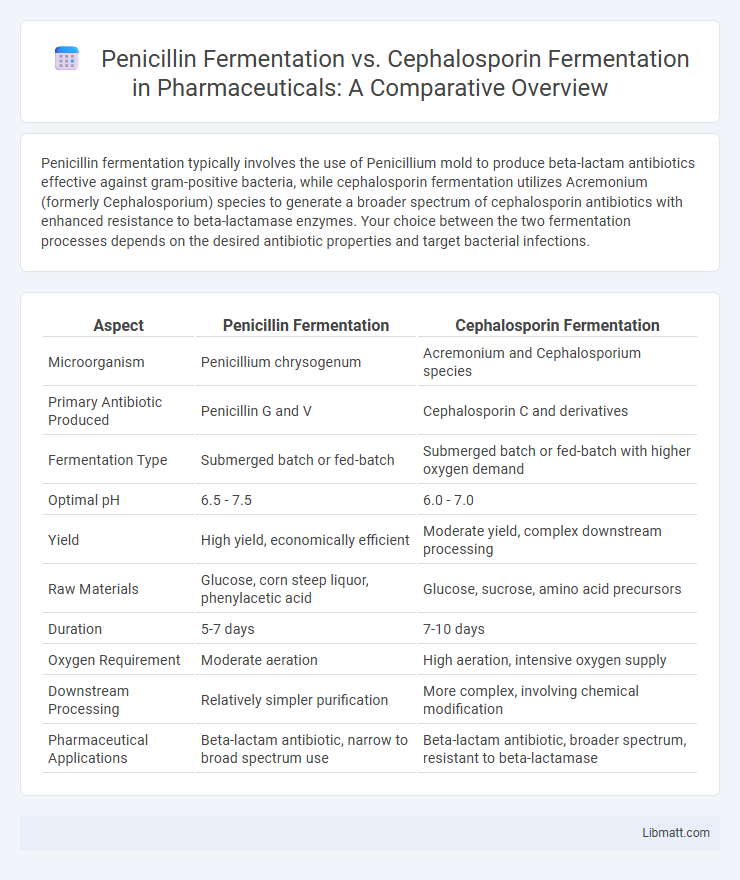
| Aspect | Penicillin Fermentation | Cephalosporin Fermentation |
|---|---|---|
| Microorganism | Penicillium chrysogenum | Acremonium and Cephalosporium species |
| Primary Antibiotic Produced | Penicillin G and V | Cephalosporin C and derivatives |
| Fermentation Type | Submerged batch or fed-batch | Submerged batch or fed-batch with higher oxygen demand |
| Optimal pH | 6.5 - 7.5 | 6.0 - 7.0 |
| Yield | High yield, economically efficient | Moderate yield, complex downstream processing |
| Raw Materials | Glucose, corn steep liquor, phenylacetic acid | Glucose, sucrose, amino acid precursors |
| Duration | 5-7 days | 7-10 days |
| Oxygen Requirement | Moderate aeration | High aeration, intensive oxygen supply |
| Downstream Processing | Relatively simpler purification | More complex, involving chemical modification |
| Pharmaceutical Applications | Beta-lactam antibiotic, narrow to broad spectrum use | Beta-lactam antibiotic, broader spectrum, resistant to beta-lactamase |
Introduction to Antibiotic Fermentation Processes
Penicillin fermentation utilizes the mold Penicillium chrysogenum to produce beta-lactam antibiotics through a deep-tank submerged fermentation process, optimizing parameters like pH, temperature, and nutrient concentration for maximal yield. Cephalosporin fermentation involves Acremonium species, converting precursor compounds into cephalosporin antibiotics with a secondary biotransformation step often requiring specific enzymes such as cephalosporin acylase. Both antibiotic fermentation processes rely on precise aerobic conditions and nutrient regulation to enhance antibiotic biosynthesis efficiency in industrial bioproduction.
Overview of Penicillin and Cephalosporin Production
Penicillin fermentation involves the cultivation of Penicillium chrysogenum under controlled conditions to produce the b-lactam antibiotic penicillin, primarily penicillin G and V, used for treating bacterial infections. Cephalosporin fermentation utilizes Acremonium (formerly Cephalosporium) molds to biosynthesize cephalosporins, a broader class of b-lactam antibiotics effective against a wider spectrum of bacteria, including penicillin-resistant strains. Both processes rely on submerged fermentation techniques but differ in microbial strains, metabolic pathways, and precursor utilization to optimize yield and antibiotic potency.
Microbial Strains Used in Penicillin and Cephalosporin Fermentation
Penicillin fermentation primarily utilizes the filamentous fungus Penicillium chrysogenum, known for its high penicillin yield and genetic adaptability. Cephalosporin fermentation relies on Acremonium chrysogenum (formerly Cephalosporium acremonium), which synthesizes cephalosporin C, the precursor to various cephalosporin antibiotics. Both microbial strains are optimized through genetic and process engineering to enhance antibiotic production efficiency and spectrum.
Substrate Selection and Medium Composition
Penicillin fermentation primarily utilizes corn steep liquor and lactose as substrates, with a medium rich in nitrogen sources and precursors like phenylacetic acid to enhance antibiotic yield. Cephalosporin fermentation requires substrates such as glucose or maltose, supplemented with specific sulfur-containing compounds like methionine to stimulate b-lactam ring biosynthesis. Medium composition for cephalosporin often includes optimized carbon-nitrogen ratios and precise pH control to maximize cefalosporin production, while penicillin focuses on maintaining oxygen levels critical for penicillin acylase activity.
Fermentation Process Conditions: Penicillin vs Cephalosporin
Penicillin fermentation typically occurs under aerobic conditions with a pH range of 6.5 to 7.5 and temperatures around 24-26degC, optimizing the growth of Penicillium chrysogenum. Cephalosporin fermentation involves a similar aerobic environment but often requires a slightly more alkaline pH of 7.0 to 7.8 and a temperature range of 25-28degC to support Acremonium chrysogenum or other producing strains. Both processes demand precise oxygen supply and nutrient control to maximize antibiotic yield but differ in microorganism requirements and subtle variations in process parameters.
Bioreactor Design and Operational Parameters
Penicillin fermentation typically uses stirred-tank bioreactors with precise control of pH around 6.5 to 7.5, temperature near 25-27degC, and oxygen transfer rates optimized for Penicillium chrysogenum growth. Cephalosporin fermentation involves Acremonium chrysogenum and often requires fed-batch or continuous bioreactors designed to maintain dissolved oxygen levels above 30% saturation and pH near 6.0 to enhance precursor conversion. Your choice of bioreactor and fine-tuning of operational parameters like agitation speed, aeration rate, and nutrient feed strategies directly impact antibiotic yield and productivity.
Metabolic Pathways: Penicillin vs Cephalosporin Biosynthesis
Penicillin biosynthesis involves the formation of the tripeptide d-(L-a-aminoadipyl)-L-cysteinyl-D-valine (ACV) catalyzed by ACV synthetase, followed by cyclization to isopenicillin N via isopenicillin N synthase, which is a pivotal step in the penicillin metabolic pathway. In contrast, cephalosporin biosynthesis shares the early ACV and isopenicillin N steps but diverges through isopenicillin N epimerase and expandase enzymes that convert isopenicillin N to penicillin N and subsequently to cephalosporin C, highlighting a more complex biosynthetic branch. The metabolic differentiation between penicillin and cephalosporin fermentation is governed by these pathway-specific enzymes that modulate the b-lactam ring structure and side-chain modifications crucial for the antibiotic activity spectrum.
Downstream Processing and Product Recovery
Downstream processing of penicillin fermentation primarily involves solvent extraction due to penicillin's sensitivity to heat and pH, facilitating efficient separation from fermentation broth while maintaining product stability. Cephalosporin fermentation downstream processing employs adsorption and ion-exchange chromatography to purify the more complex cephalosporin molecules, enhancing yield and purity in the recovery stage. Both processes require careful pH control and membrane filtration to remove impurities, but cephalosporin demands more rigorous conditions due to its structural complexity and susceptibility to degradation.
Yield Optimization and Productivity Challenges
Penicillin fermentation typically achieves higher yield optimization through the use of genetically improved strains of Penicillium chrysogenum and carefully controlled substrate feeding strategies, whereas cephalosporin fermentation involves more complex precursor feeding and multi-stage fermentation processes that present significant productivity challenges. The productivity in penicillin fermentation is enhanced by optimizing parameters like dissolved oxygen, pH, and nutrient availability, while cephalosporin fermentation faces difficulties due to the lower conversion efficiency of cephalosporin precursors and the need for maintaining sterile conditions over extended periods. Industrial-scale production of cephalosporins often requires advanced bioprocess engineering and enzyme catalysis techniques to overcome fermentation bottlenecks and improve overall product yield.
Comparative Analysis: Industrial Applications and Future Trends
Penicillin fermentation primarily utilizes Penicillium chrysogenum in submerged fermentation processes to produce beta-lactam antibiotics with broad-spectrum antibacterial activity, widely applied in treating gram-positive infections. Cephalosporin fermentation typically employs Acremonium species, producing cephalosporins with enhanced resistance to beta-lactamases, making them crucial in combating resistant bacterial strains, especially in hospital settings. Your strategic focus on optimizing fermentation parameters and genetic engineering advances can drive future trends towards higher yield, novel cephalosporin derivatives, and sustainable bioprocess scalability in pharmaceutical industries.
Penicillin Fermentation vs Cephalosporin Fermentation Infographic
 libmatt.com
libmatt.com