USP Class VI focuses primarily on the biocompatibility and safety of materials used in medical devices through in vivo testing, while ISO 10993 provides a comprehensive framework for evaluating the biological risks of medical devices, including cytotoxicity, sensitization, and systemic toxicity. Understanding the differences between USP Class VI and ISO 10993 helps you choose the appropriate testing standards to ensure device safety and regulatory compliance.
Table of Comparison
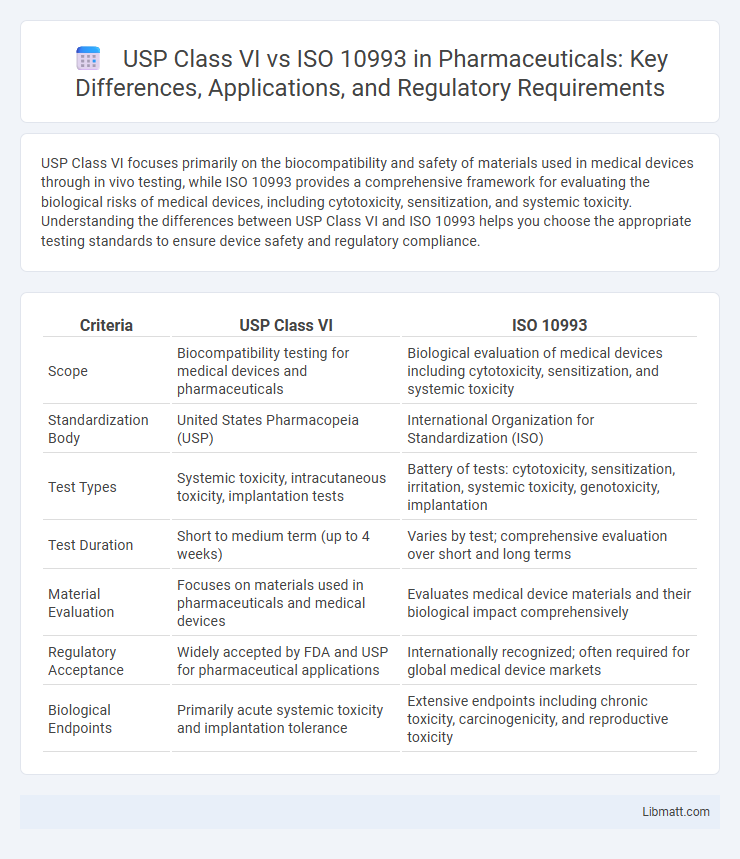
| Criteria | USP Class VI | ISO 10993 |
|---|---|---|
| Scope | Biocompatibility testing for medical devices and pharmaceuticals | Biological evaluation of medical devices including cytotoxicity, sensitization, and systemic toxicity |
| Standardization Body | United States Pharmacopeia (USP) | International Organization for Standardization (ISO) |
| Test Types | Systemic toxicity, intracutaneous toxicity, implantation tests | Battery of tests: cytotoxicity, sensitization, irritation, systemic toxicity, genotoxicity, implantation |
| Test Duration | Short to medium term (up to 4 weeks) | Varies by test; comprehensive evaluation over short and long terms |
| Material Evaluation | Focuses on materials used in pharmaceuticals and medical devices | Evaluates medical device materials and their biological impact comprehensively |
| Regulatory Acceptance | Widely accepted by FDA and USP for pharmaceutical applications | Internationally recognized; often required for global medical device markets |
| Biological Endpoints | Primarily acute systemic toxicity and implantation tolerance | Extensive endpoints including chronic toxicity, carcinogenicity, and reproductive toxicity |
Understanding USP Class VI and ISO 10993: An Overview
USP Class VI sets stringent biocompatibility standards for medical devices through a series of biological reactivity tests on plastic materials, ensuring safety for implantable and prolonged contact applications. ISO 10993 offers a comprehensive framework for evaluating the biocompatibility of medical devices, encompassing cytotoxicity, sensitization, and systemic toxicity across multiple parts tailored to different materials and device types. Both standards are critical in regulatory approval processes, with USP Class VI focusing on in vitro and in vivo tests for plastics, while ISO 10993 provides a broader assessment applicable to diverse medical device components.
Historical Background and Development
USP Class VI originated in the 1970s as part of the United States Pharmacopeia's efforts to standardize biocompatibility testing for medical devices, emphasizing systemic toxicity, intracutaneous toxicity, and implantation tests. ISO 10993 was developed later by the International Organization for Standardization, beginning in the 1990s, to provide a comprehensive framework for evaluating the biocompatibility of medical devices across multiple biological endpoints. The evolution of ISO 10993 reflects a broader international consensus and integration of advances in toxicology, whereas USP Class VI remains a more specific, historically rooted standard primarily recognized in the U.S. regulatory context.
Scope of Applicability in Medical Device Industry
USP Class VI certification focuses primarily on the biocompatibility of materials used in medical devices through standardized in vivo testing, ensuring they meet strict toxicity requirements for implantable and surface-contacting applications. ISO 10993 covers a broader range of biological evaluations, including cytotoxicity, sensitization, and hemocompatibility, applicable to various device types and materials throughout the entire product lifecycle. Understanding the scope of each standard helps you select the appropriate testing protocols to ensure safety and regulatory compliance for your specific medical device.
Testing Requirements and Methodologies
USP Class VI testing emphasizes biological evaluation of medical devices through systemic injection, intracutaneous, and implantation tests to assess toxicity and biocompatibility in vivo. ISO 10993 provides a comprehensive framework for evaluating biocompatibility via a suite of tests including cytotoxicity, sensitization, irritation, and systemic toxicity, tailored to device type and contact duration. Both standards require strict adherence to sample preparation, extraction procedures, and validated test methodologies to ensure reliable assessment of material safety.
Biological Evaluation Criteria
USP Class VI and ISO 10993 differ in their biological evaluation criteria, with USP Class VI focusing on systemic toxicity, intracutaneous toxicity, and implantation tests to ensure materials are safe for medical use. ISO 10993 provides a broader framework assessing biocompatibility through multiple endpoints such as cytotoxicity, sensitization, genotoxicity, and chronic toxicity, tailored to the specific device and exposure routes. Understanding these criteria helps you select the appropriate testing strategy for regulatory compliance and patient safety.
Regulatory Acceptance and Global Perspectives
USP Class VI and ISO 10993 are critical standards for biocompatibility testing in medical devices, with USP Class VI widely accepted by the FDA for implantable materials and ISO 10993 serving as the international benchmark for biological evaluation. Regulatory acceptance varies as USP Class VI primarily addresses specific tests such as systemic toxicity, intracutaneous reactivity, and implantation studies, while ISO 10993 offers a comprehensive framework covering cytotoxicity, sensitization, and genotoxicity across global markets including the EU, Japan, and China. Understanding these distinctions is essential for manufacturers targeting regulatory approval, ensuring compliance with region-specific requirements and facilitating global market access.
Material Selection Considerations
Material selection for medical devices under USP Class VI emphasizes stringent biological reactivity tests to ensure biocompatibility, including systemic toxicity, intracutaneous toxicity, and implantation assessments. ISO 10993 offers a comprehensive framework evaluating cytotoxicity, sensitization, and genotoxicity, allowing tailored testing based on device type and contact duration. Selecting materials requires understanding both standards' specific test requirements and clinical application to optimize biocompatibility and regulatory compliance.
Certification Process: Steps and Documentation
USP Class VI certification requires rigorous biological evaluation involving tests for systemic toxicity, intracutaneous reactivity, and implantation, with detailed documentation including test protocols, results, and material composition. The ISO 10993 certification process involves a comprehensive risk assessment plan, selection of relevant biocompatibility tests based on device type and usage, and submission of a technical file containing test reports, chemical characterization, and toxicological risk assessment. Your documentation must be thorough and precise to meet regulatory standards and ensure the safety and compliance of medical devices under both USP Class VI and ISO 10993 frameworks.
Pros and Cons: USP Class VI vs ISO 10993
USP Class VI testing provides rigorous biological evaluation focused primarily on systemic toxicity, intracutaneous reactivity, and implantation, offering a streamlined approach for medical devices needing FDA clearance. However, it lacks comprehensive coverage of all biocompatibility endpoints, which ISO 10993 addresses through a more extensive framework encompassing cytotoxicity, sensitization, genotoxicity, and long-term implantation effects. While USP Class VI tests are faster and more cost-effective, ISO 10993 offers broader risk assessment crucial for regulatory approval across global markets.
Making the Right Choice for Compliance
Selecting between USP Class VI and ISO 10993 hinges on the specific regulatory requirements and the intended use of medical devices. USP Class VI certification focuses on biological reactivity tests for plastics, such as systemic toxicity and intracutaneous toxicity, ensuring materials are safe for prolonged tissue contact. ISO 10993 offers a comprehensive risk assessment framework covering cytotoxicity, sensitization, genotoxicity, and more, making it ideal for thorough biocompatibility evaluation and global compliance.
USP Class VI vs ISO 10993 Infographic
 libmatt.com
libmatt.com