Microbial Limit Test detects and quantifies the presence of specific microorganisms within a product, ensuring it meets defined microbial limits, while Sterility Test verifies the complete absence of viable microorganisms in sterile pharmaceutical products. Understanding the differences helps you select the appropriate testing method to ensure product safety and compliance with regulatory standards.
Table of Comparison
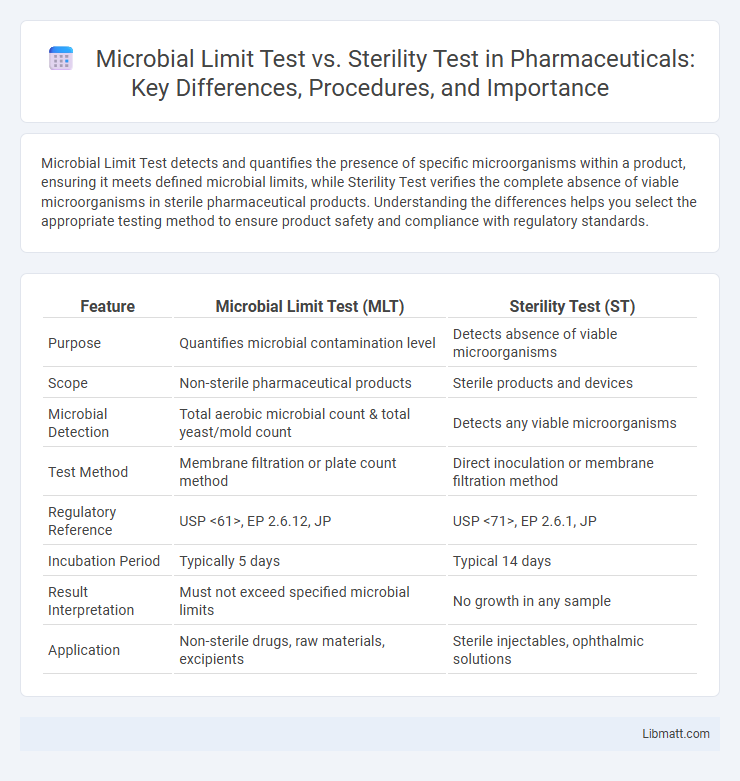
| Feature | Microbial Limit Test (MLT) | Sterility Test (ST) |
|---|---|---|
| Purpose | Quantifies microbial contamination level | Detects absence of viable microorganisms |
| Scope | Non-sterile pharmaceutical products | Sterile products and devices |
| Microbial Detection | Total aerobic microbial count & total yeast/mold count | Detects any viable microorganisms |
| Test Method | Membrane filtration or plate count method | Direct inoculation or membrane filtration method |
| Regulatory Reference | USP <61>, EP 2.6.12, JP | USP <71>, EP 2.6.1, JP |
| Incubation Period | Typically 5 days | Typical 14 days |
| Result Interpretation | Must not exceed specified microbial limits | No growth in any sample |
| Application | Non-sterile drugs, raw materials, excipients | Sterile injectables, ophthalmic solutions |
Introduction to Microbial Limit Test and Sterility Test
Microbial Limit Test evaluates the total viable microbial count and specifies the presence of objectionable microorganisms in pharmaceutical products, ensuring they meet safety standards. Sterility Test determines the absence of all viable microorganisms in sterile products, confirming the product is free from contamination. Your choice between these tests depends on the product type and regulatory requirements for microbial quality assurance.
Definitions and Key Concepts
Microbial Limit Test detects and quantifies specific microorganisms in non-sterile products to ensure safety and compliance with pharmacopeial standards, focusing on total aerobic microbial count and total yeast and mold count. Sterility Test determines the absence of viable microorganisms in sterile products, verifying their sterility according to regulatory requirements by using suitable culture media and incubation conditions. Both tests are critical for quality control in pharmaceutical manufacturing but target different product categories and microbiological risks.
Purpose of Microbial Limit Test
The purpose of the Microbial Limit Test is to ensure that pharmaceutical products, raw materials, and finished goods meet specified microbiological quality standards by detecting and quantifying the presence of specified microorganisms. This test helps to verify the safety and cleanliness of non-sterile products by identifying microbial contamination levels. It plays a crucial role in quality control by preventing the distribution of products that exceed acceptable microbial limits.
Purpose of Sterility Test
Sterility test aims to confirm the absence of viable microorganisms in pharmaceutical products, ensuring product safety and compliance with regulatory standards. This test is critical for sterile products such as injectables, surgical implants, and ophthalmic solutions that must be free from contamination to prevent infections. By detecting contamination, sterility testing helps maintain product efficacy and protects patient health.
Differences in Testing Procedures
The Microbial Limit Test involves quantifying and identifying the presence of specific microorganisms within a sample, using methods like membrane filtration or plate count techniques to ensure contamination levels stay within acceptable limits. In contrast, the Sterility Test aims to confirm the complete absence of viable microorganisms by incubating the product in nutrient media under controlled conditions for an extended period, typically 14 days. Your choice between these tests depends on whether detection of low-level contamination or absolute sterility verification is required for product safety compliance.
Regulatory Guidelines and Standards
Regulatory guidelines for Microbial Limit Tests are primarily outlined in pharmacopeias like USP <61> and EP 2.6.12, specifying acceptable microbial counts for non-sterile products, ensuring microbial contamination is within safe limits. Sterility Tests follow stricter standards such as USP <71> and EP 2.6.1, mandating the complete absence of viable microorganisms in sterile products to guarantee safety and efficacy, with regulatory agencies enforcing these protocols to maintain product integrity.
Sample Types and Applications
Microbial Limit Test is primarily applied to non-sterile pharmaceutical samples such as oral tablets, ointments, and topical solutions to detect and quantify microbial contamination within specified limits. Sterility Test targets sterile products including injectables, ophthalmic solutions, and surgical implants to confirm the complete absence of viable microorganisms. Both tests ensure product safety but differ in sample types and regulatory requirements based on the intended use and risk associated with microbial presence.
Interpretation of Results
Microbial Limit Test results indicate the presence and quantity of specific microorganisms within acceptable limits, ensuring that the microbial contamination does not exceed established thresholds. Sterility Test results confirm the complete absence of viable microorganisms, indicating that a product is sterile and safe for use, especially in critical pharmaceutical applications. Your choice between these tests depends on whether you need to quantify microbial levels or verify absolute sterility for regulatory compliance and product safety.
Common Challenges and Limitations
Microbial Limit Test (MLT) and Sterility Test both face common challenges such as detecting low levels of microbial contamination in pharmaceutical products with high sensitivity and specificity. Limitations include the inability of MLT to identify non-culturable or slow-growing organisms and the Sterility Test's extended incubation times, which may delay product release. Variability in sample size, potential false positives from environmental contaminants, and method validation complexities further complicate accurate microbial assessment.
Choosing the Right Test for Your Product
Choosing the right test for your product depends on its intended use and regulatory requirements. Microbial Limit Tests are designed to quantify and limit the number of viable microorganisms in non-sterile products, ensuring they meet safety standards. Sterility Tests are essential for products that must be free from all viable microorganisms, like injectable pharmaceuticals, providing assurance of aseptic manufacturing and product safety.
Microbial Limit Test vs Sterility Test Infographic
 libmatt.com
libmatt.com