NIR spectroscopy provides rapid, non-destructive analysis by measuring overtone and combination vibrations of molecular bonds, making it ideal for assessing moisture and organic compounds. Raman spectroscopy offers detailed molecular fingerprinting through inelastic scattering of light, enabling precise identification of chemical structures in your samples.
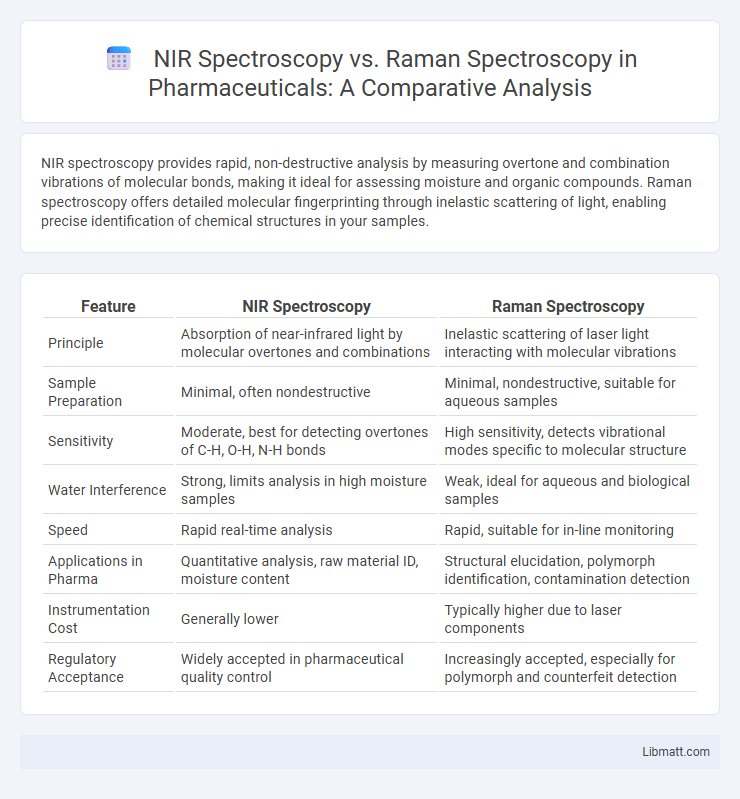
Table of Comparison
| Feature | NIR Spectroscopy | Raman Spectroscopy |
|---|---|---|
| Principle | Absorption of near-infrared light by molecular overtones and combinations | Inelastic scattering of laser light interacting with molecular vibrations |
| Sample Preparation | Minimal, often nondestructive | Minimal, nondestructive, suitable for aqueous samples |
| Sensitivity | Moderate, best for detecting overtones of C-H, O-H, N-H bonds | High sensitivity, detects vibrational modes specific to molecular structure |
| Water Interference | Strong, limits analysis in high moisture samples | Weak, ideal for aqueous and biological samples |
| Speed | Rapid real-time analysis | Rapid, suitable for in-line monitoring |
| Applications in Pharma | Quantitative analysis, raw material ID, moisture content | Structural elucidation, polymorph identification, contamination detection |
| Instrumentation Cost | Generally lower | Typically higher due to laser components |
| Regulatory Acceptance | Widely accepted in pharmaceutical quality control | Increasingly accepted, especially for polymorph and counterfeit detection |
Introduction to Spectroscopic Techniques
NIR spectroscopy measures molecular vibrations by detecting overtones and combinations of bond vibrations primarily in the near-infrared region, making it ideal for analyzing organic compounds and water content. Raman spectroscopy relies on inelastic scattering of monochromatic light to provide detailed molecular fingerprints based on vibrational modes, useful for identifying chemical structures and crystalline forms. Your choice between these techniques depends on specific sample properties, desired information, and environmental conditions.
Fundamentals of NIR Spectroscopy
NIR spectroscopy relies on the absorption of near-infrared light by molecular overtones and combinations of fundamental vibrations, primarily involving C-H, N-H, and O-H bonds. This technique probes vibrational transitions that occur at higher energy levels than those detected in Raman spectroscopy, offering unique insights into molecular composition and concentration. Your analytical applications benefit from NIR's non-destructive nature and rapid data acquisition, making it ideal for quantifying organic components in diverse samples.
Basics of Raman Spectroscopy
Raman spectroscopy is a vibrational spectroscopic technique that measures inelastic scattering of monochromatic light, usually from a laser, resulting in energy shifts corresponding to molecular vibrational modes. It complements Near-Infrared (NIR) spectroscopy by providing detailed information about molecular vibrations and chemical structure through Raman-active molecular bond interactions. Raman spectroscopy is highly sensitive to polarizability changes in molecular bonds, enabling identification of functional groups and molecular conformations in complex samples.
Key Differences Between NIR and Raman Spectroscopy
NIR spectroscopy measures the overtone and combination vibrations of molecular bonds using near-infrared light, making it highly effective for analyzing bulk materials and organic compounds. Raman spectroscopy detects inelastic scattering of monochromatic light to provide detailed molecular fingerprint information, ideal for studying chemical composition and molecular structure. Your choice depends on sample type, sensitivity requirements, and specific application needs, with NIR excelling in rapid, non-destructive analysis and Raman offering precise molecular insights.
Sample Preparation and Measurement Conditions
NIR spectroscopy requires minimal sample preparation and works effectively with diffuse reflectance or transmission modes, making it ideal for rapid, non-destructive analysis of powders, liquids, and solids. Raman spectroscopy demands careful control of sample lighting and temperature to minimize fluorescence interference and obtain clear vibrational spectra, often requiring little to no chemical treatment but precise laser calibration. Your choice depends on the sample type and environment, as NIR excels in moisture and bulk component analysis while Raman offers detailed molecular insights under controlled measurement conditions.
Sensitivity and Selectivity Comparison
NIR spectroscopy exhibits high sensitivity to overtone and combination vibrations, making it effective for bulk composition analysis but often suffers from overlapping bands, reducing selectivity. Raman spectroscopy provides enhanced selectivity by detecting fundamental vibrational modes with sharper, well-resolved peaks, enabling precise identification of molecular structures even in complex mixtures. Sensitivity in Raman can be lower due to weak scattering signals, but techniques like Surface-Enhanced Raman Scattering (SERS) significantly improve detection limits for trace analysis.
Applications in Pharmaceutical and Chemical Analysis
NIR spectroscopy offers rapid, non-destructive analysis ideal for monitoring pharmaceutical formulation, content uniformity, and moisture content, enhancing quality control and process optimization. Raman spectroscopy excels in chemical characterization and polymorph identification, providing detailed molecular information crucial for detecting impurities and studying drug-excipient interactions. Both techniques complement each other in pharmaceutical and chemical analysis by delivering precise, real-time data to ensure product efficacy and safety.
Advantages and Limitations of NIR Spectroscopy
NIR spectroscopy offers rapid, non-destructive analysis with deep sample penetration and minimal sample preparation, making it ideal for quality control in pharmaceuticals, agriculture, and food industries. It provides good quantitative results for organic compounds but suffers from overlapping broad bands and lower molecular specificity compared to Raman spectroscopy. Limited sensitivity to low-concentration analytes and interference from water absorption are notable limitations of NIR spectroscopy in complex sample matrices.
Strengths and Weaknesses of Raman Spectroscopy
Raman spectroscopy excels in providing detailed molecular fingerprinting with minimal sample preparation and is highly effective for analyzing aqueous samples due to its weak water interference. However, it is limited by fluorescence interference, which can overwhelm the Raman signal, and typically requires longer acquisition times compared to NIR spectroscopy. You should consider Raman spectroscopy when precise chemical characterization is essential, despite its sensitivity to sample fluorescence and lower speed.
Choosing the Right Technique for Your Analytical Needs
NIR spectroscopy excels in rapid, non-destructive analysis of bulk samples and moisture content, making it ideal for quality control in pharmaceuticals and agriculture. Raman spectroscopy offers detailed molecular fingerprinting, enabling precise identification of chemical structures and detection of low-concentration compounds in complex matrices. Your choice depends on factors like sample type, desired information depth, and environmental conditions, with NIR suited for routine quantitative analysis and Raman preferred for molecular specificity.
NIR spectroscopy vs Raman spectroscopy Infographic
 libmatt.com
libmatt.com