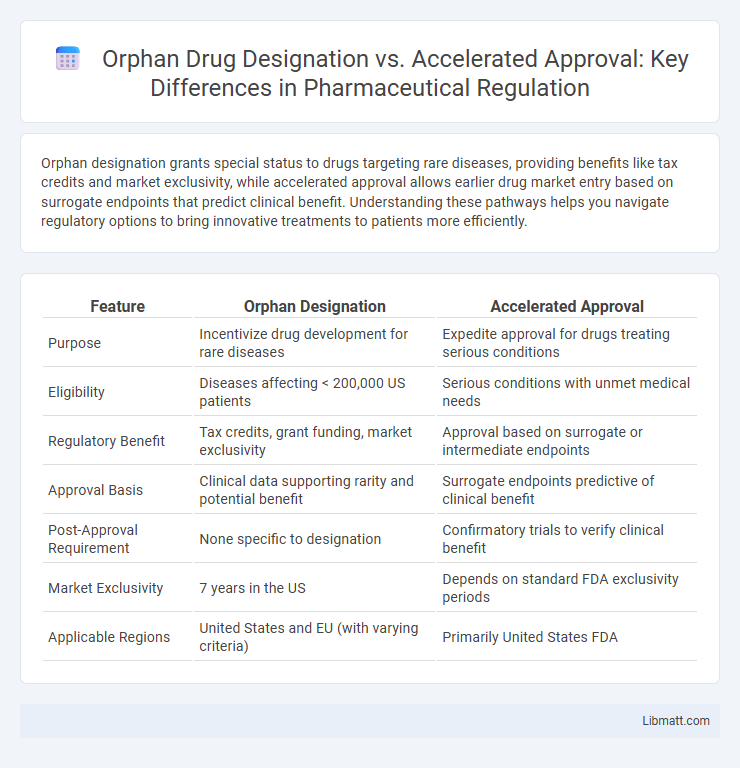
Orphan designation grants special status to drugs targeting rare diseases, providing benefits like tax credits and market exclusivity, while accelerated approval allows earlier drug market entry based on surrogate endpoints that predict clinical benefit. Understanding these pathways helps you navigate regulatory options to bring innovative treatments to patients more efficiently.
Table of Comparison
| Feature | Orphan Designation | Accelerated Approval |
|---|---|---|
| Purpose | Incentivize drug development for rare diseases | Expedite approval for drugs treating serious conditions |
| Eligibility | Diseases affecting < 200,000 US patients | Serious conditions with unmet medical needs |
| Regulatory Benefit | Tax credits, grant funding, market exclusivity | Approval based on surrogate or intermediate endpoints |
| Approval Basis | Clinical data supporting rarity and potential benefit | Surrogate endpoints predictive of clinical benefit |
| Post-Approval Requirement | None specific to designation | Confirmatory trials to verify clinical benefit |
| Market Exclusivity | 7 years in the US | Depends on standard FDA exclusivity periods |
| Applicable Regions | United States and EU (with varying criteria) | Primarily United States FDA |
Introduction to Orphan Designation and Accelerated Approval
Orphan Designation is a regulatory status granted to drugs targeting rare diseases affecting fewer than 200,000 patients in the U.S., providing incentives like tax credits and market exclusivity to encourage development. Accelerated Approval allows earlier approval of drugs for serious conditions based on surrogate endpoints reasonably likely to predict clinical benefit, expediting patient access. Both pathways aim to address unmet medical needs but differ in scope: Orphan Designation targets rare diseases, while Accelerated Approval focuses on speed to market for life-threatening conditions.
Definitions: Orphan Designation Explained
Orphan Designation is a regulatory status granted to drugs intended to treat rare diseases affecting fewer than 200,000 patients in the United States. This designation provides incentives such as market exclusivity, tax credits, and fee waivers to encourage drug development for rare conditions. Unlike Accelerated Approval, which speeds up the marketing of drugs based on surrogate endpoints for serious conditions, Orphan Designation specifically targets the economic challenges of developing treatments for rare diseases.
Understanding Accelerated Approval Pathway
The Accelerated Approval pathway allows treatments for serious conditions that fill an unmet medical need to gain earlier approval based on surrogate or intermediate clinical endpoints. This regulatory mechanism is distinct from Orphan Designation, which targets drugs for rare diseases but does not inherently speed the approval process. You should understand that Accelerated Approval can expedite patient access to promising therapies while requiring post-marketing confirmatory trials.
Key Regulatory Differences: Orphan vs Accelerated Approval
Orphan designation grants incentives for developing treatments for rare diseases affecting fewer than 200,000 patients in the U.S., including tax credits, user fee waivers, and market exclusivity for seven years. Accelerated approval allows earlier drug approval based on surrogate endpoints that predict clinical benefit, primarily for serious or life-threatening conditions, enabling faster patient access while requiring confirmatory post-marketing trials. Unlike orphan designation, accelerated approval does not confer market exclusivity but focuses on shortening development timelines through regulatory flexibility.
Eligibility Criteria for Orphan Designation
Orphan designation eligibility requires a condition affecting fewer than 200,000 people in the U.S. or one lacking sufficient profitability without incentives. The drug must demonstrate potential for diagnosing, preventing, or treating a rare disease or condition. This designation offers benefits such as tax credits, grant funding, and market exclusivity to encourage development.
Eligibility Requirements for Accelerated Approval
Accelerated approval is designed for drugs treating serious or life-threatening conditions with an unmet medical need, requiring evidence that the drug has an effect on a surrogate or intermediate clinical endpoint reasonably likely to predict clinical benefit. Sponsors must conduct post-marketing confirmatory trials to verify and describe the drug's anticipated clinical benefit. Eligibility excludes drugs with adequate and well-controlled studies demonstrating clinical benefit unless the new indication offers significant advantages over existing therapies.
Benefits of Orphan Designation for Drug Developers
Orphan designation provides drug developers with benefits such as market exclusivity for seven years, tax credits for clinical trial costs, and eligibility for grant funding, significantly reducing financial risk. This designation facilitates faster development timelines by enabling protocol assistance and regulatory support from agencies like the FDA. You gain strategic advantages that improve the feasibility of bringing treatments for rare diseases to market.
Advantages and Limitations of Accelerated Approval
Accelerated approval offers the advantage of faster access to promising treatments by allowing earlier market entry based on surrogate endpoints, which can significantly benefit patients with serious conditions. However, this pathway carries limitations such as the requirement for post-approval confirmatory trials to verify clinical benefits, and the risk that some therapies may not ultimately demonstrate the expected efficacy. Your awareness of these benefits and challenges can help in making informed decisions about treatment options under accelerated approval.
Real-World Examples: Orphan Drugs vs Accelerated Approvals
Orphan designation granted to rare disease treatments like Spinraza for spinal muscular atrophy highlights targeted development incentives without guaranteed expedited market entry. Accelerated approval, as seen with Aduhelm for Alzheimer's disease, allows earlier patient access based on surrogate endpoints, often requiring post-approval confirmatory trials. Real-world examples reveal orphan drugs often benefit from market exclusivity and tax credits, while accelerated approvals emphasize earlier availability amid ongoing evidence gathering.
Strategic Considerations for Regulatory Pathway Selection
Strategic considerations for regulatory pathway selection involve balancing orphan designation benefits, such as market exclusivity and fee reductions, against accelerated approval's opportunity for earlier market access based on surrogate endpoints. Evaluating the target patient population size, clinical data robustness, and development timelines helps determine the optimal pathway to maximize both regulatory incentives and time-to-market advantages. Understanding specific regulatory requirements and alignment with business goals ensures efficient resource allocation and successful product commercialization.
Orphan designation vs accelerated approval Infographic
 libmatt.com
libmatt.com