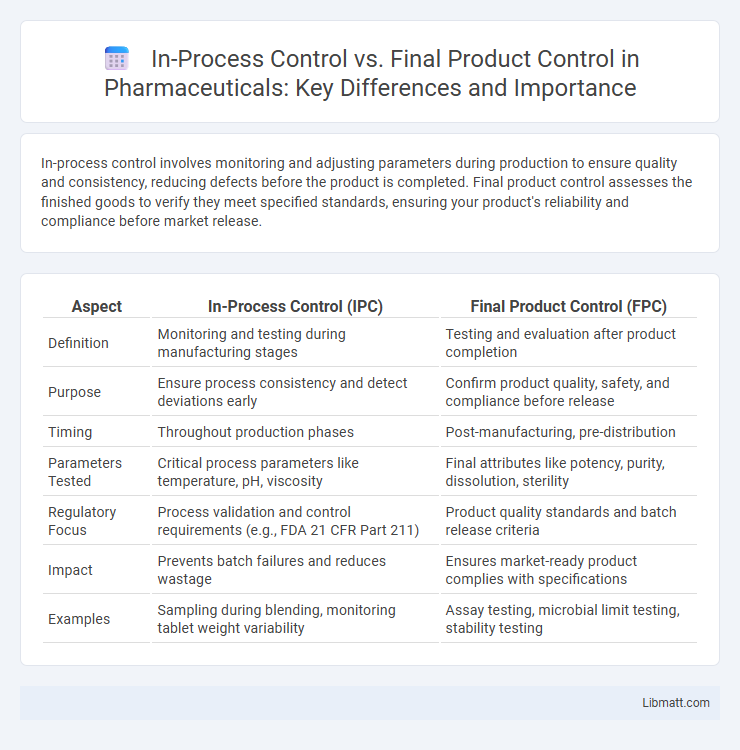
In-process control involves monitoring and adjusting parameters during production to ensure quality and consistency, reducing defects before the product is completed. Final product control assesses the finished goods to verify they meet specified standards, ensuring your product's reliability and compliance before market release.
Table of Comparison
| Aspect | In-Process Control (IPC) | Final Product Control (FPC) |
|---|---|---|
| Definition | Monitoring and testing during manufacturing stages | Testing and evaluation after product completion |
| Purpose | Ensure process consistency and detect deviations early | Confirm product quality, safety, and compliance before release |
| Timing | Throughout production phases | Post-manufacturing, pre-distribution |
| Parameters Tested | Critical process parameters like temperature, pH, viscosity | Final attributes like potency, purity, dissolution, sterility |
| Regulatory Focus | Process validation and control requirements (e.g., FDA 21 CFR Part 211) | Product quality standards and batch release criteria |
| Impact | Prevents batch failures and reduces wastage | Ensures market-ready product complies with specifications |
| Examples | Sampling during blending, monitoring tablet weight variability | Assay testing, microbial limit testing, stability testing |
Introduction to Quality Control in Manufacturing
In-process control in manufacturing involves continuous monitoring and inspection during production to detect and correct deviations early, ensuring product consistency and reducing waste. Final product control refers to the comprehensive evaluation of finished goods to verify compliance with quality standards before market release. Effective quality control combines in-process measures with final product testing to maintain high manufacturing standards and customer satisfaction.
Defining In-Process Control
In-process control refers to the continuous monitoring and evaluation of critical parameters during manufacturing to ensure product quality and compliance with specifications. This approach enables early detection of deviations, reducing risk of defects before the final product stage. By maintaining strict control over raw materials, equipment settings, and processing conditions, in-process control ensures consistent production output aligned with quality standards.
Understanding Final Product Control
Final product control ensures that the finished goods meet all quality standards and regulatory requirements before reaching the customer, verifying attributes such as safety, functionality, and compliance. This stage involves rigorous testing and inspection methods to detect defects or deviations that may have been missed during in-process control. Understanding final product control is crucial for maintaining your brand reputation and preventing non-compliant products from entering the market.
Key Objectives of In-Process Control
In-process control aims to monitor and regulate production parameters in real-time to ensure consistent product quality and early detection of deviations. It focuses on critical attributes such as temperature, pH, and moisture content to maintain process stability and prevent defects. These controls significantly reduce waste and enhance compliance with regulatory standards by enabling timely corrective actions.
Main Goals of Final Product Control
Final product control primarily aims to ensure that the finished goods meet predefined quality standards and comply with regulatory requirements before reaching the consumer. It focuses on verifying product specifications, detecting defects, and confirming safety and efficacy through rigorous testing and inspection. This step serves as the final checkpoint to prevent non-conforming products from entering the market, thereby safeguarding brand reputation and customer satisfaction.
Comparative Analysis: In-Process vs Final Product Control
In-process control involves continuous monitoring and adjustments during production to ensure quality parameters meet specifications, reducing defects early and minimizing rework. Final product control focuses on inspecting finished goods to verify compliance with quality standards before market release, serving as a last checkpoint to prevent nonconforming products. In-process control offers proactive quality assurance with real-time data, whereas final product control provides retrospective verification, making their combined application crucial for comprehensive quality management.
Benefits of Implementing In-Process Control
In-process control enhances product quality by enabling real-time monitoring and immediate correction during manufacturing, reducing defects and waste. This proactive approach ensures consistency and compliance with regulatory standards, lowering the risk of costly recalls. Integrating in-process control into your production line improves efficiency and reliability, ultimately boosting customer satisfaction.
Limitations of Relying on Final Product Control
Final product control alone cannot detect variability or defects occurring during different production stages, increasing the risk of defective batches reaching consumers. It often results in delayed identification of quality issues that could have been corrected earlier in the manufacturing process. This limitation underscores the necessity of integrating in-process control to ensure consistent product quality and reduce waste.
Industry Best Practices for Quality Assurance
Industry best practices for quality assurance emphasize in-process control to detect and correct defects during production, minimizing waste and ensuring consistent quality. Final product control serves as a crucial validation step, verifying that the finished goods meet regulatory standards and customer specifications before shipment. Combining both controls enhances overall product reliability, reduces risk, and supports compliance with ISO 9001 and FDA guidelines.
Future Trends in Process and Product Quality Control
In-process control leverages real-time data analytics and AI-driven sensors to monitor manufacturing variables continuously, enhancing product consistency and reducing defects before finalization. Final product control is evolving through advanced non-destructive testing and machine learning algorithms that predict long-term quality and performance based on comprehensive post-production analysis. Your ability to integrate these future trends ensures higher efficiency, reduced waste, and smarter quality assurance throughout the production lifecycle.
In-process control vs final product control Infographic
 libmatt.com
libmatt.com