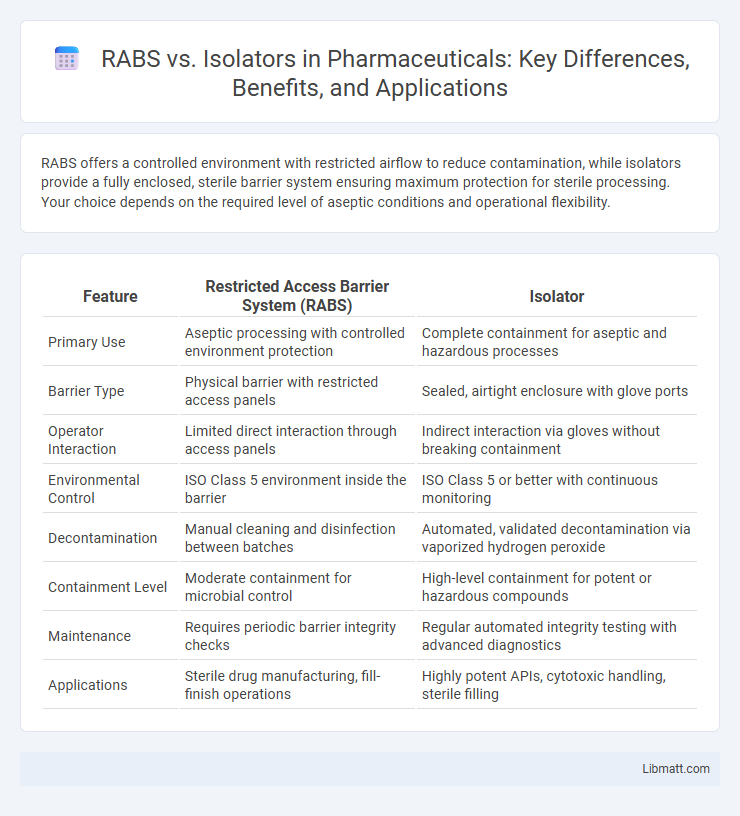
RABS offers a controlled environment with restricted airflow to reduce contamination, while isolators provide a fully enclosed, sterile barrier system ensuring maximum protection for sterile processing. Your choice depends on the required level of aseptic conditions and operational flexibility.
Table of Comparison
| Feature | Restricted Access Barrier System (RABS) | Isolator |
|---|---|---|
| Primary Use | Aseptic processing with controlled environment protection | Complete containment for aseptic and hazardous processes |
| Barrier Type | Physical barrier with restricted access panels | Sealed, airtight enclosure with glove ports |
| Operator Interaction | Limited direct interaction through access panels | Indirect interaction via gloves without breaking containment |
| Environmental Control | ISO Class 5 environment inside the barrier | ISO Class 5 or better with continuous monitoring |
| Decontamination | Manual cleaning and disinfection between batches | Automated, validated decontamination via vaporized hydrogen peroxide |
| Containment Level | Moderate containment for microbial control | High-level containment for potent or hazardous compounds |
| Maintenance | Requires periodic barrier integrity checks | Regular automated integrity testing with advanced diagnostics |
| Applications | Sterile drug manufacturing, fill-finish operations | Highly potent APIs, cytotoxic handling, sterile filling |
Introduction to RABS and Isolators
Restricted Access Barrier Systems (RABS) and isolators are advanced containment technologies designed to protect sterile environments in pharmaceutical manufacturing. RABS provide a physical barrier with limited operator access through gloves or sleeves, while isolators offer a fully enclosed environment with complete operator separation. Both systems enhance contamination control but differ in operational flexibility and level of sterility assurance.
Key Definitions: What are RABS and Isolators?
Restricted Access Barrier Systems (RABS) are specially designed enclosures that provide a controlled environment to minimize contamination risk by limiting operator interaction in pharmaceutical manufacturing. Isolators are fully sealed systems with a physical barrier and glove ports, offering a higher level of protection by completely isolating the product from the external environment. Both technologies are critical for aseptic processing but differ in their containment and operational complexity.
Core Components and Design Features
RABS (Restricted Access Barrier Systems) feature glove ports and transparent barriers that limit operator access while maintaining an aseptic environment, focusing on physical separation to reduce contamination risk. Isolators incorporate a fully enclosed design with built-in HEPA filtration and pressure differentials, ensuring a controlled environment through rigid containment and enhanced sterility assurance. Your choice between RABS and isolators depends on the required level of contamination control and operational complexity.
Containment Levels and Environmental Control
RABS (Restricted Access Barrier Systems) offer intermediate containment levels designed to reduce operator exposure and product contamination, while isolators provide the highest containment with complete physical separation between the operator and the process. Environmental control in isolators is more stringent, maintaining ISO Class 5 conditions inside the barrier with controlled pressure differentials, whereas RABS rely on regular cleanroom conditions combined with partial physical barriers. Your choice depends on the required aseptic standards and risk assessment for contamination and operator safety.
Operational Process Differences
RABS (Restricted Access Barrier Systems) and isolators differ significantly in their operational processes; RABS rely on physical barriers and glove ports to restrict operator access while allowing interventions, whereas isolators provide complete containment with sealed, negative pressure environments minimizing contamination risk. Your choice affects workflow complexity, as RABS permit easier manual handling contrasted with isolators' fully automated, controlled processes requiring validated sterilization cycles. These distinctions influence cleanroom classifications and compliance with regulatory standards like GMP and FDA guidelines for sterile manufacturing.
Cleaning, Sterilization, and Maintenance
RABS (Restricted Access Barrier System) and Isolators differ significantly in cleaning, sterilization, and maintenance processes, with Isolators offering superior environmental control due to their sealed design that minimizes contamination risk. RABS require frequent manual cleaning and sterilization of the open transfer areas, whereas Isolators use automated sterilization methods like vaporized hydrogen peroxide, reducing operator intervention and contamination. Your choice impacts operational efficiency and compliance, as Isolators generally demand less maintenance and provide more consistent sterility assurance compared to RABS.
Regulatory Compliance and Industry Standards
RABS (Restricted Access Barrier Systems) and isolators both comply with stringent regulatory standards such as FDA, EMA, and USP <797> and <800>, ensuring sterile manufacturing environments. Isolators provide superior containment and environmental control, meeting higher biosafety and contamination prevention requirements compared to RABS. Your facility's choice between RABS and isolators should consider specific regulatory expectations and industry standards for aseptic processing and operator safety.
Cost Implications and Implementation
RABS (Restricted Access Barrier Systems) generally have lower initial investment and installation costs compared to isolators, making them suitable for facilities with budget constraints. Isolators require higher capital expenditure due to complex engineering, advanced automation, and stringent maintenance demands, but offer superior contamination control and operational efficiency. Implementation timelines for RABS are typically shorter, while isolators demand extensive validation and staff training, impacting total project duration and operational readiness.
Advantages and Limitations of RABS
Restricted Access Barrier Systems (RABS) offer enhanced contamination control with reduced operational complexity compared to isolators, providing easy operator access and flexible intervention during aseptic processing. Their main advantages include lower initial investment costs, simplified maintenance, and improved ergonomic conditions for operators. However, RABS have limitations such as lower containment integrity than isolators, increased risk of operator-related contamination, and dependence on strict environmental controls to maintain sterility.
Advantages and Limitations of Isolators
Isolators provide superior contamination control by creating a physical barrier between operators and the product, reducing the risk of microbial and particulate contamination in aseptic processing. Their sealed environment offers enhanced sterility assurance compared to RABS, allowing for longer batch processing times and fewer interventions. However, isolators can be more complex and costly to install and maintain, requiring specialized training and validation efforts to ensure optimal performance.
RABS vs Isolator Infographic
 libmatt.com
libmatt.com