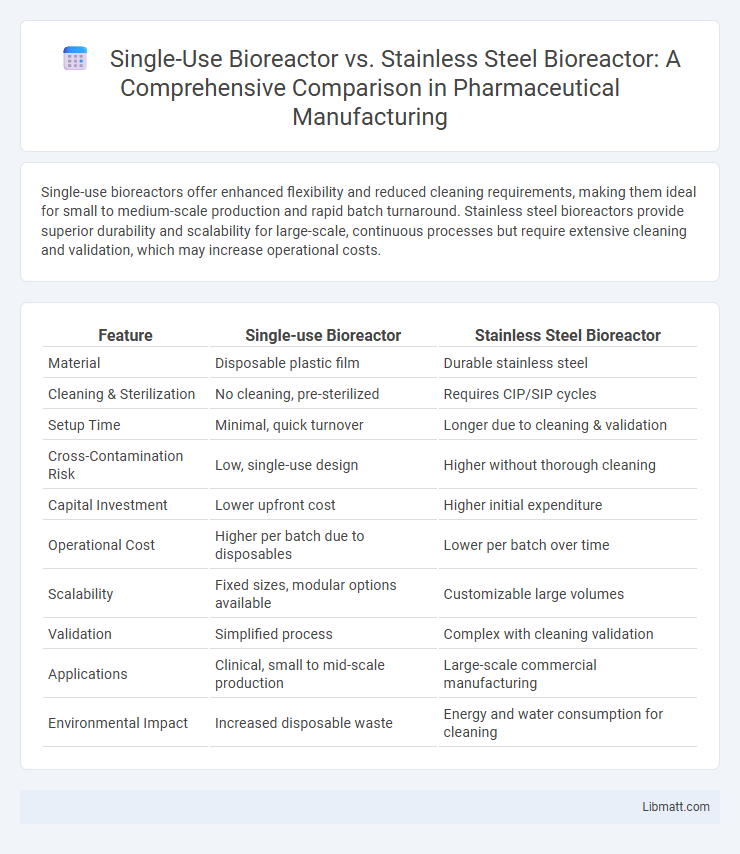
Single-use bioreactors offer enhanced flexibility and reduced cleaning requirements, making them ideal for small to medium-scale production and rapid batch turnaround. Stainless steel bioreactors provide superior durability and scalability for large-scale, continuous processes but require extensive cleaning and validation, which may increase operational costs.
Table of Comparison
| Feature | Single-use Bioreactor | Stainless Steel Bioreactor |
|---|---|---|
| Material | Disposable plastic film | Durable stainless steel |
| Cleaning & Sterilization | No cleaning, pre-sterilized | Requires CIP/SIP cycles |
| Setup Time | Minimal, quick turnover | Longer due to cleaning & validation |
| Cross-Contamination Risk | Low, single-use design | Higher without thorough cleaning |
| Capital Investment | Lower upfront cost | Higher initial expenditure |
| Operational Cost | Higher per batch due to disposables | Lower per batch over time |
| Scalability | Fixed sizes, modular options available | Customizable large volumes |
| Validation | Simplified process | Complex with cleaning validation |
| Applications | Clinical, small to mid-scale production | Large-scale commercial manufacturing |
| Environmental Impact | Increased disposable waste | Energy and water consumption for cleaning |
Introduction to Bioreactor Technologies
Single-use bioreactors utilize disposable plastic bags, reducing cross-contamination risks and cleaning requirements, thus enabling faster turnaround times in bioprocessing. Stainless steel bioreactors offer durability, precise control over process parameters, and are commonly used for large-scale, long-term production in pharmaceutical manufacturing. The choice between these bioreactor technologies impacts manufacturing efficiency, scalability, and cost-effectiveness in biopharmaceutical development.
Overview of Single-use Bioreactors
Single-use bioreactors (SUBs) offer a flexible and cost-effective solution for biopharmaceutical production by eliminating the need for cleaning and sterilization between batches. These systems utilize disposable plastic bags, reducing the risk of cross-contamination and allowing rapid changeover in multi-product facilities. Single-use bioreactors support scalable cell culture processes with volumes typically ranging from 50 liters to 2,000 liters, making them ideal for clinical and commercial production phases.
Key Features of Stainless Steel Bioreactors
Stainless steel bioreactors offer exceptional durability, corrosion resistance, and the ability to withstand high temperatures and pressures, ensuring robust performance in large-scale industrial processes. Their customizable design supports complex control systems for precise monitoring and optimization of fermentation parameters, enhancing product consistency and yield. Your choice of stainless steel bioreactor enables thorough cleaning and sterilization protocols, reducing contamination risks and supporting long-term operational reliability.
Cost Comparison: Initial Investment and Operating Expenses
Single-use bioreactors typically require lower initial investment costs due to minimal infrastructure and faster setup times compared to stainless steel bioreactors, which demand substantial capital for fabrication and clean-in-place systems. Operating expenses for single-use systems often include higher consumable costs and waste disposal fees, whereas stainless steel bioreactors incur ongoing expenses related to cleaning, sterilization, maintenance, and utility consumption. Cost efficiency ultimately depends on production scale, batch frequency, and process flexibility, with single-use bioreactors favoring smaller, multi-product operations and stainless steel systems benefiting large-scale, continuous manufacturing.
Scalability and Flexibility in Manufacturing
Single-use bioreactors offer superior scalability through modular design, enabling rapid expansion without the need for extensive facility modifications, which reduces downtime and capital investment. Stainless steel bioreactors provide robust flexibility for large-scale production with consistent ruggedness, but scaling up often requires significant infrastructure changes and longer turnaround times for cleaning and validation. Manufacturing operations prioritize single-use systems for their adaptability in multi-product environments, while stainless steel remains favored for high-volume, long-term continuous processes.
Sterilization and Contamination Risks
Single-use bioreactors eliminate the need for traditional sterilization methods by using pre-sterilized, disposable components, significantly reducing the risk of contamination between batches. Stainless steel bioreactors require rigorous cleaning and steam-in-place (SIP) sterilization processes, which can be time-consuming and have higher contamination risk if protocols are not strictly followed. The single-use design offers enhanced contamination control through single-use bags and tubing, minimizing cross-contamination and improving operational efficiency in bioprocessing.
Environmental Impact and Sustainability
Single-use bioreactors significantly reduce water and energy consumption compared to stainless steel bioreactors, as they eliminate the need for extensive cleaning and sterilization processes. The disposable nature of single-use systems generates more plastic waste, but advances in recycling and biodegradable materials are mitigating environmental concerns. Stainless steel bioreactors offer durability and reusability, promoting long-term sustainability but often at the cost of higher resource use and carbon footprint during manufacturing and operation.
Process Control and Performance
Single-use bioreactors offer enhanced process control through pre-sterilized, disposable components that reduce contamination risk and streamline setup time, enabling rapid batch-to-batch consistency. Stainless steel bioreactors provide robust performance with advanced monitoring systems for precise control of parameters like pH, temperature, and dissolved oxygen, supporting large-scale production and long-term use. Your choice depends on flexibility needs and scale, with single-use systems excelling in agility and stainless steel in durability and process precision.
Regulatory Compliance Considerations
Single-use bioreactors offer enhanced regulatory compliance due to reduced risk of cross-contamination and simpler validation processes compared to stainless steel bioreactors, which require extensive cleaning and sterilization validation to meet stringent FDA and EMA standards. Your choice between these systems impacts adherence to cGMP guidelines, where single-use systems streamline batch records and lower contamination control risks. Regulatory agencies often prefer single-use bioreactors for flexible manufacturing but demand comprehensive risk assessments for material compatibility and extractables.
Choosing the Right Bioreactor for Your Application
Selecting the right bioreactor between single-use and stainless steel depends on production scale, flexibility, and contamination control requirements. Single-use bioreactors offer reduced cleaning and faster turnaround times, ideal for smaller batches and multi-product facilities, while stainless steel bioreactors provide durability and cost-efficiency in large-scale continuous production. Evaluate factors such as capital investment, maintenance, and process validation to optimize bioprocessing outcomes.
Single-use Bioreactor vs Stainless Steel Bioreactor Infographic
 libmatt.com
libmatt.com