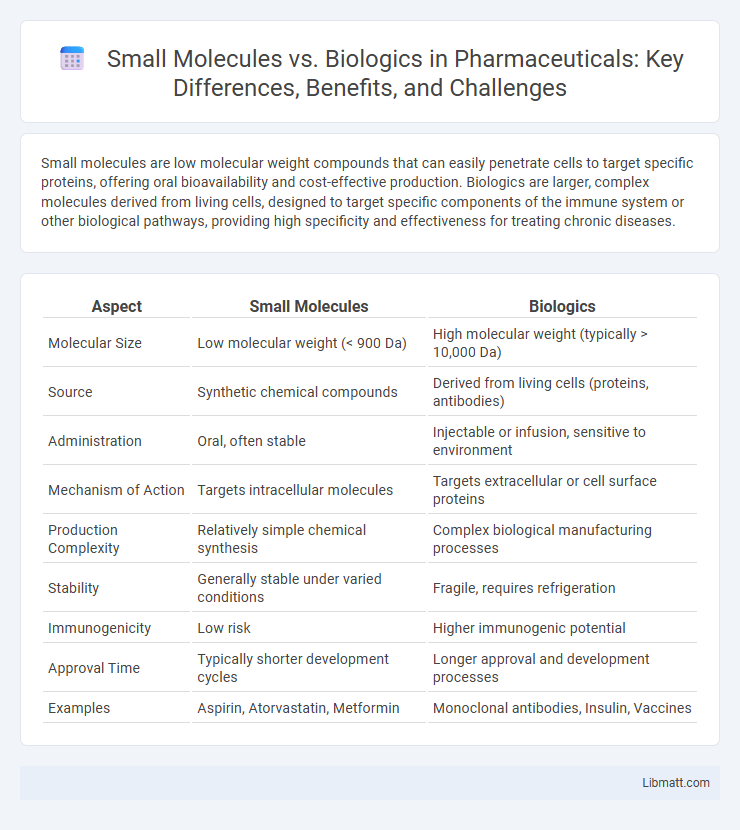
Small molecules are low molecular weight compounds that can easily penetrate cells to target specific proteins, offering oral bioavailability and cost-effective production. Biologics are larger, complex molecules derived from living cells, designed to target specific components of the immune system or other biological pathways, providing high specificity and effectiveness for treating chronic diseases.
Table of Comparison
| Aspect | Small Molecules | Biologics |
|---|---|---|
| Molecular Size | Low molecular weight (< 900 Da) | High molecular weight (typically > 10,000 Da) |
| Source | Synthetic chemical compounds | Derived from living cells (proteins, antibodies) |
| Administration | Oral, often stable | Injectable or infusion, sensitive to environment |
| Mechanism of Action | Targets intracellular molecules | Targets extracellular or cell surface proteins |
| Production Complexity | Relatively simple chemical synthesis | Complex biological manufacturing processes |
| Stability | Generally stable under varied conditions | Fragile, requires refrigeration |
| Immunogenicity | Low risk | Higher immunogenic potential |
| Approval Time | Typically shorter development cycles | Longer approval and development processes |
| Examples | Aspirin, Atorvastatin, Metformin | Monoclonal antibodies, Insulin, Vaccines |
Introduction to Small Molecules and Biologics
Small molecules are low molecular weight compounds that easily penetrate cells, allowing them to target intracellular processes and receptors. Biologics consist of large, complex proteins or nucleic acids derived from living organisms, designed to interact with specific extracellular targets such as antibodies or cytokines. Understanding the fundamental differences between small molecules and biologics helps optimize your therapeutic strategies based on molecular size, mechanism of action, and manufacturing complexity.
Defining Small Molecules: Structure and Characteristics
Small molecules are low molecular weight compounds, typically under 900 daltons, characterized by their simple, well-defined chemical structures that enable cell membrane permeability and oral bioavailability. These small organic compounds interact with specific biological targets by binding to active sites or enzymes, often acting as inhibitors or modulators. Their stable chemical nature and ease of synthesis differentiate them from larger, complex biologics used in targeted therapies.
What Are Biologics? Composition and Complexity
Biologics are large, complex molecules derived from living cells, including proteins, antibodies, and nucleic acids, designed to target specific components of the immune system or disease pathways. Unlike small molecules, biologics have intricate three-dimensional structures that are sensitive to manufacturing conditions, making their production highly specialized and tightly regulated. Your choice between small molecules and biologics depends on the disease mechanism, as biologics often offer targeted therapy with potentially fewer side effects due to their sophisticated composition.
Mechanisms of Action: Small Molecules vs Biologics
Small molecules typically function by penetrating cell membranes to interact with intracellular targets such as enzymes or receptors, thereby modulating biochemical pathways. Biologics, including monoclonal antibodies and recombinant proteins, primarily target extracellular molecules or cell surface receptors with high specificity, often triggering immune responses or blocking ligand-receptor interactions. The distinct mechanisms of action between small molecules and biologics influence their pharmacokinetics, therapeutic applications, and safety profiles in drug development.
Manufacturing Processes: Chemical Synthesis versus Biotech Production
Small molecule drugs are manufactured through chemical synthesis, involving precise, scalable chemical reactions to produce well-defined, low molecular weight compounds. Biologics rely on biotech production methods, utilizing living cells such as bacteria, yeast, or mammalian cells to express complex proteins, antibodies, or nucleic acids in bioreactors. The manufacturing of biologics demands stringent control of cell culture conditions, purification, and quality assurance to maintain product consistency and bioactivity.
Routes of Administration and Dosage Forms
Small molecules are typically administered orally in tablet or capsule form due to their ability to withstand the digestive environment and achieve systemic absorption. Biologics, composed of large protein structures, require parenteral routes such as intravenous, subcutaneous, or intramuscular injections to ensure bioavailability and stability. Dosage forms for biologics include prefilled syringes, auto-injectors, and infusion bags, contrasting with the more convenient and varied oral, topical, or inhalation dosage forms available for small molecules.
Clinical Applications: Therapeutic Areas and Indications
Small molecules are widely used in therapeutic areas such as cardiovascular diseases, oncology, and infectious diseases due to their ability to penetrate cells and modulate intracellular targets. Biologics primarily target complex conditions like autoimmune disorders, cancers, and rare genetic diseases by interacting with specific proteins or cells in the immune system. Your treatment choice may depend on the disease indication, molecular target, and desired immune response modulation.
Safety, Side Effects, and Immunogenicity
Small molecules generally exhibit lower immunogenicity compared to biologics, making them less likely to trigger immune responses or allergic reactions. Biologics, derived from complex proteins, carry higher risks of immunogenicity that can lead to serious side effects or safety concerns. Understanding these distinctions helps you weigh treatment options based on safety profiles and potential adverse effects.
Regulatory Pathways and Approval Processes
Small molecules typically undergo an Abbreviated New Drug Application (ANDA) or New Drug Application (NDA) through the FDA, emphasizing well-established synthetic manufacturing processes and clear pharmacokinetic profiles. Biologics follow the Biologics License Application (BLA) pathway, under the stricter regulatory oversight due to their complex structure, manufacturing variability, and immunogenicity potential. Your development strategy should account for these distinct regulatory requirements to ensure efficient approval and market entry.
Future Trends: Innovations and Emerging Therapies
Future trends in small molecule and biologic therapies highlight innovations such as targeted drug delivery systems, personalized medicine, and advances in gene editing technologies like CRISPR. Emerging therapies include bispecific antibodies, antibody-drug conjugates, and small molecule inhibitors designed for complex diseases like cancer and autoimmune disorders. Your treatment options will increasingly benefit from these innovations, offering enhanced efficacy and reduced side effects.
Small molecule vs Biologics Infographic
 libmatt.com
libmatt.com