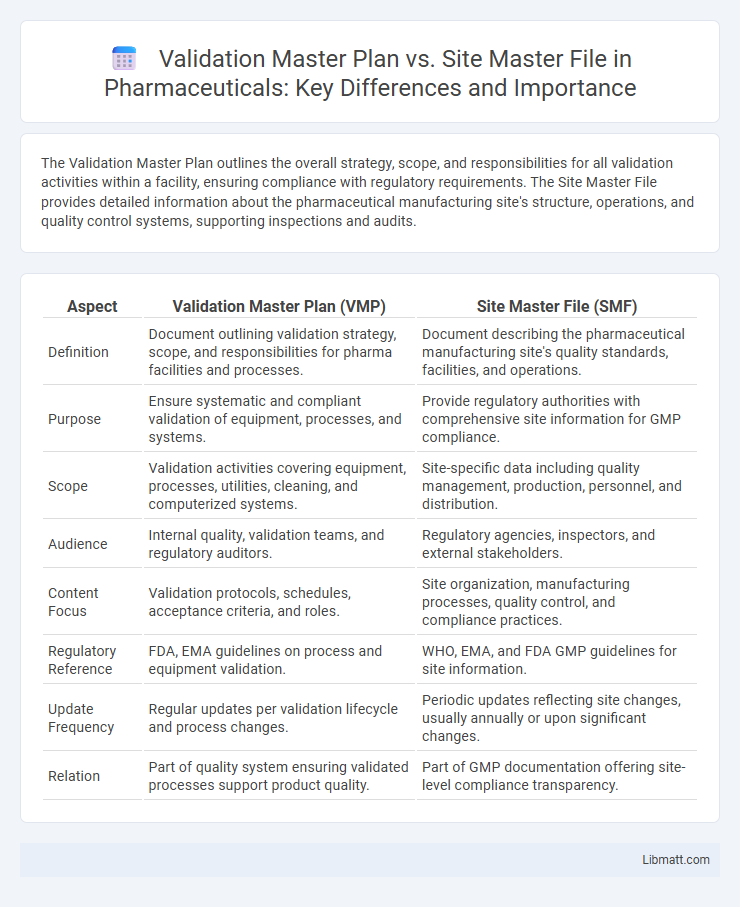
The Validation Master Plan outlines the overall strategy, scope, and responsibilities for all validation activities within a facility, ensuring compliance with regulatory requirements. The Site Master File provides detailed information about the pharmaceutical manufacturing site's structure, operations, and quality control systems, supporting inspections and audits.
Table of Comparison
| Aspect | Validation Master Plan (VMP) | Site Master File (SMF) |
|---|---|---|
| Definition | Document outlining validation strategy, scope, and responsibilities for pharma facilities and processes. | Document describing the pharmaceutical manufacturing site's quality standards, facilities, and operations. |
| Purpose | Ensure systematic and compliant validation of equipment, processes, and systems. | Provide regulatory authorities with comprehensive site information for GMP compliance. |
| Scope | Validation activities covering equipment, processes, utilities, cleaning, and computerized systems. | Site-specific data including quality management, production, personnel, and distribution. |
| Audience | Internal quality, validation teams, and regulatory auditors. | Regulatory agencies, inspectors, and external stakeholders. |
| Content Focus | Validation protocols, schedules, acceptance criteria, and roles. | Site organization, manufacturing processes, quality control, and compliance practices. |
| Regulatory Reference | FDA, EMA guidelines on process and equipment validation. | WHO, EMA, and FDA GMP guidelines for site information. |
| Update Frequency | Regular updates per validation lifecycle and process changes. | Periodic updates reflecting site changes, usually annually or upon significant changes. |
| Relation | Part of quality system ensuring validated processes support product quality. | Part of GMP documentation offering site-level compliance transparency. |
Introduction to Validation Master Plan and Site Master File
Validation Master Plan (VMP) outlines a comprehensive strategy for validating manufacturing processes, equipment, and systems to ensure consistent product quality and regulatory compliance. Site Master File (SMF) provides a detailed overview of a pharmaceutical manufacturing site's quality management system, facilities, personnel, and production capabilities. Your understanding of both documents is critical for maintaining GMP standards and facilitating regulatory inspections.
Purpose and Scope of Validation Master Plan
The Validation Master Plan (VMP) outlines the overall strategy, scope, and responsibilities for validating processes, equipment, and systems to ensure compliance with regulatory requirements and product quality. It provides a comprehensive framework for planning, executing, and documenting validation activities across the entire manufacturing lifecycle. In contrast, the Site Master File (SMF) serves as a detailed overview of the manufacturing site's facilities, operations, and quality management systems, primarily supporting regulatory inspections rather than detailing validation strategies.
Purpose and Scope of Site Master File
The Site Master File (SMF) documents comprehensive information about a manufacturing site, including quality management systems, personnel qualifications, facility descriptions, and production processes to ensure regulatory compliance and product quality. Its purpose is to provide regulatory authorities with a clear overview of the site's operations, resource allocation, and adherence to Good Manufacturing Practices (GMP). The scope of the SMF encompasses the entire manufacturing facility and supports site-specific regulatory inspections and audits, distinguishing it from the Validation Master Plan, which focuses specifically on validation activities and protocols.
Key Components of a Validation Master Plan
A Validation Master Plan (VMP) outlines the comprehensive strategy, objectives, scope, and responsibilities for validating facilities, systems, and processes within pharmaceutical manufacturing, ensuring compliance with regulatory standards like FDA and EMA. Essential components include validation scope, validation approach, risk assessments, schedule of validation activities, and documentation requirements. Unlike the Site Master File (SMF), which provides an overview of the manufacturing site, personnel, and quality management systems, the VMP specifically governs the methodical validation processes critical for product quality assurance.
Key Elements of a Site Master File
A Site Master File (SMF) details essential aspects of a pharmaceutical manufacturing site, including organizational structure, quality management systems, facility description, equipment, production processes, and personnel qualifications. Key elements of an SMF ensure compliance with Good Manufacturing Practices (GMP) and regulatory requirements by providing comprehensive site information for regulatory authorities. Unlike a Validation Master Plan, which focuses on the planned validation activities and strategy, the SMF emphasizes the overall site operations and infrastructure supporting product quality and safety.
Regulatory Requirements: VMP vs SMF
The Validation Master Plan (VMP) and Site Master File (SMF) both address critical regulatory requirements but serve distinct purposes; the VMP outlines the strategy and scope for validating manufacturing processes per FDA and EMA guidelines, ensuring compliance with GxP standards. The SMF provides comprehensive documentation of the site's quality management system, facilities, and operations, aligning with WHO, PIC/S, and ICH Q7 regulations for site qualification and GMP adherence. Regulatory authorities expect the VMP to detail validation protocols and timelines, while the SMF supports inspections by describing organizational structure and site-specific quality practices.
Differences Between VMP and SMF
The Validation Master Plan (VMP) outlines the overall strategy, scope, and responsibilities for validation activities within a pharmaceutical or manufacturing facility, focusing on ensuring compliance with regulatory requirements. In contrast, the Site Master File (SMF) provides a comprehensive overview of the manufacturing site's facilities, quality management system, production processes, and personnel qualifications. While the VMP is primarily concerned with validation protocols and documentation control, the SMF serves as a reference document for auditors and regulatory authorities about the site's operational and organizational structure.
Similarities and Overlapping Areas
Validation Master Plan (VMP) and Site Master File (SMF) both serve as critical documentation frameworks in pharmaceutical manufacturing, ensuring regulatory compliance and quality assurance. Both documents include detailed information on facility operations, quality management systems, and validation activities, demonstrating the commitment to Good Manufacturing Practices (GMP). Overlapping areas emphasize the integration of validation strategies with site-specific processes, highlighting shared responsibilities in equipment qualification, process validation, and documentation control.
Best Practices for Developing VMP and SMF
Developing a Validation Master Plan (VMP) involves systematically outlining validation activities, timelines, responsibilities, and documentation requirements to ensure compliance with regulatory standards such as FDA 21 CFR Part 210/211 and EU GMP Annex 15. Best practices for creating a Site Master File (SMF) emphasize comprehensive documentation of site-specific information, including quality management systems, personnel qualifications, manufacturing processes, and facility descriptions, aligning with WHO and EMA guidelines. Integrating cross-referenced data between the VMP and SMF enhances traceability, facilitates regulatory audits, and supports a robust quality management system essential for pharmaceutical manufacturing compliance.
Importance of VMP and SMF in Pharmaceutical Compliance
Validation Master Plan (VMP) and Site Master File (SMF) are critical documents ensuring pharmaceutical compliance by outlining validation strategies and operational details respectively. VMP provides a comprehensive roadmap for validation activities, guaranteeing processes meet regulatory standards and product quality requirements, while the SMF offers essential site-specific information that supports regulatory inspections and audits. Your adherence to both VMP and SMF is vital for maintaining regulatory compliance, product safety, and operational transparency within pharmaceutical manufacturing.
Validation master plan vs Site master file Infographic
 libmatt.com
libmatt.com