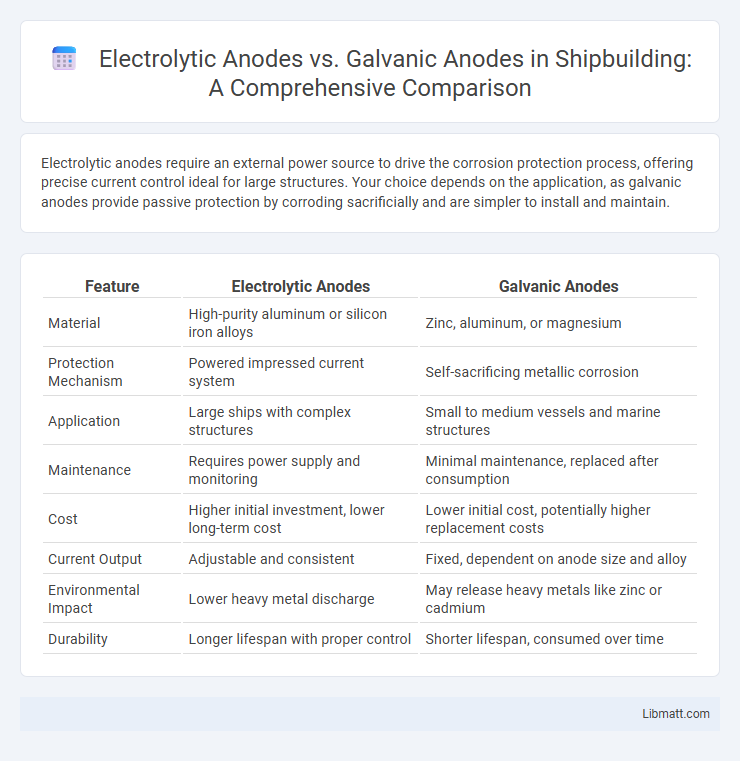
Electrolytic anodes require an external power source to drive the corrosion protection process, offering precise current control ideal for large structures. Your choice depends on the application, as galvanic anodes provide passive protection by corroding sacrificially and are simpler to install and maintain.
Table of Comparison
| Feature | Electrolytic Anodes | Galvanic Anodes |
|---|---|---|
| Material | High-purity aluminum or silicon iron alloys | Zinc, aluminum, or magnesium |
| Protection Mechanism | Powered impressed current system | Self-sacrificing metallic corrosion |
| Application | Large ships with complex structures | Small to medium vessels and marine structures |
| Maintenance | Requires power supply and monitoring | Minimal maintenance, replaced after consumption |
| Cost | Higher initial investment, lower long-term cost | Lower initial cost, potentially higher replacement costs |
| Current Output | Adjustable and consistent | Fixed, dependent on anode size and alloy |
| Environmental Impact | Lower heavy metal discharge | May release heavy metals like zinc or cadmium |
| Durability | Longer lifespan with proper control | Shorter lifespan, consumed over time |
Introduction to Anodes in Corrosion Protection
Electrolytic anodes and galvanic anodes serve crucial roles in corrosion protection by preventing metal structures from deteriorating in aggressive environments. Electrolytic anodes require an external power source to supply current, enabling controlled cathodic protection for large-scale applications like pipelines and storage tanks. Your choice between these anode types depends on factors such as installation complexity, maintenance needs, and the specific environment where corrosion protection is essential.
Understanding Electrolytic Anodes
Electrolytic anodes operate using an external power source to supply a controlled electrical current, enabling precise protection against corrosion in structures like pipelines and storage tanks. They offer adjustable current output, making them ideal for large-scale or complex systems where protection levels vary over time. Unlike galvanic anodes that rely on natural electrochemical potential differences, electrolytic anodes provide consistent and efficient cathodic protection through active current control.
What Are Galvanic Anodes?
Galvanic anodes are sacrificial metal components, typically made from zinc, magnesium, or aluminum alloys, used to protect metal structures from corrosion through cathodic protection. These anodes function by corroding preferentially, thereby preventing the deterioration of the protected metal surface, commonly employed in marine environments, pipelines, and underground tanks. Their efficiency depends on the potential difference between the anode and the structure, enabling a consistent flow of protective current without external power sources.
Electrolytic vs Galvanic Anodes: Key Differences
Electrolytic anodes require an external power source to provide a controlled current flow for corrosion protection, whereas galvanic anodes rely on the natural electrochemical potential difference between the anode and the metal structure. Electrolytic anodes offer adjustable current output and are ideal for complex or large-scale cathodic protection systems, while galvanic anodes function passively, making them simpler and cost-effective for smaller or isolated applications. The choice between electrolytic and galvanic anodes depends on factors such as installation environment, maintenance requirements, and system design requirements.
Mechanisms of Corrosion Control
Electrolytic anodes control corrosion by using an external power source to supply a continuous protective current that counteracts the electrochemical reactions causing metal degradation, ensuring precise and adjustable corrosion prevention. Galvanic anodes rely on the natural electrochemical difference between the anode material and the protected structure, sacrificing themselves to divert corrosive reactions away from your metal assets. Both mechanisms effectively prevent corrosion but are chosen based on factors like structure size, environment, and current requirements for optimal protection.
Material Composition of Anodes
Electrolytic anodes primarily consist of high-purity lead, tin, or mixed metal oxides, optimized for stability and controlled current output in impressed current cathodic protection systems. Galvanic anodes are typically made from more active metals such as zinc, magnesium, or aluminum alloys, which provide a natural electrochemical potential to protect structures through sacrificial corrosion. The distinct material compositions directly impact their application suitability, lifespan, and the magnitude of protective current generated.
Installation and Maintenance Requirements
Electrolytic anodes require a power source for installation, involving more complex wiring and equipment setup compared to galvanic anodes, which are simpler and self-powered due to their metal composition. Maintenance of electrolytic anodes demands regular electrical system checks and monitoring to ensure proper function, while galvanic anodes need periodic physical inspections and replacements as they corrode over time. Your choice should consider the ease of installation and ongoing maintenance, especially in environments where accessibility is limited.
Cost Comparison and Longevity
Electrolytic anodes generally involve higher initial costs due to their power supply requirements but offer longer longevity by providing controlled and consistent current output, making them cost-effective over time. Galvanic anodes have lower upfront costs and simpler installation but tend to have shorter service life, requiring more frequent replacement and higher maintenance expenses. Choosing the right anode depends on balancing your budget constraints with the expected lifespan and performance needs of the corrosion protection system.
Best Applications for Each Anode Type
Electrolytic anodes are best suited for impressed current cathodic protection systems in large industrial applications such as pipelines, storage tanks, and offshore structures due to their high efficiency and controllable current output. Galvanic anodes excel in small-scale or remote environments like underground water pipes, ships, and marine structures where simplicity and maintenance-free operation are priorities, relying on the natural electrochemical potential difference. Selecting the appropriate anode depends on factors including current requirements, environmental conditions, and installation complexity.
How to Choose the Right Anode for Your Project
Choosing between electrolytic anodes and galvanic anodes depends on your project's specific requirements such as current output, environmental conditions, and maintenance needs. Electrolytic anodes provide controllable current output suitable for large or complex systems requiring precise corrosion protection, while galvanic anodes rely on natural electrochemical potential differences, making them ideal for simpler, smaller-scale applications with limited power sources. Your decision should factor in the compatibility of the anode material with the structure, the electrolyte environment, and installation constraints to ensure optimal cathodic protection performance.
Electrolytic anodes vs galvanic anodes Infographic
 libmatt.com
libmatt.com