Alcoholic fermentation converts sugars into ethanol and carbon dioxide using yeast, commonly utilized in brewing and winemaking. Acetic fermentation transforms ethanol into acetic acid through acetic acid bacteria, essential for producing vinegar.
Table of Comparison
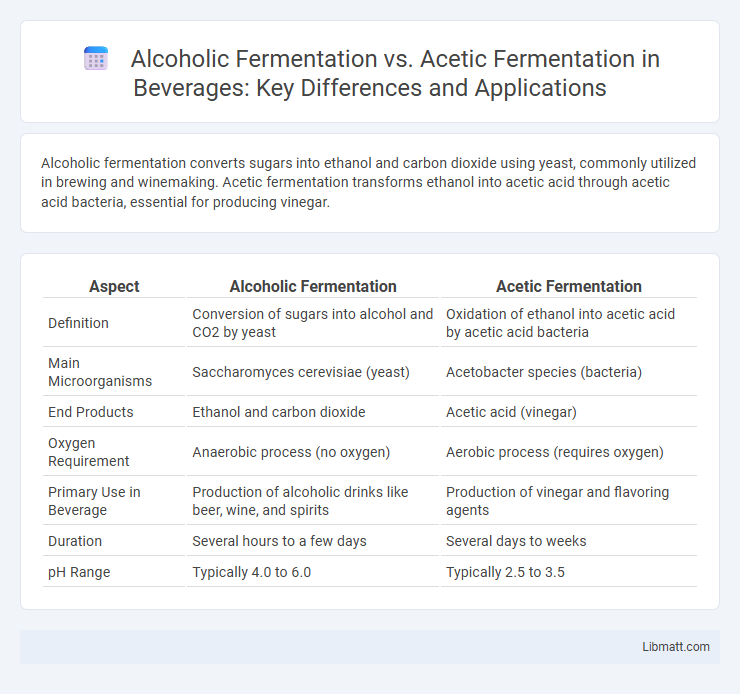
| Aspect | Alcoholic Fermentation | Acetic Fermentation |
|---|---|---|
| Definition | Conversion of sugars into alcohol and CO2 by yeast | Oxidation of ethanol into acetic acid by acetic acid bacteria |
| Main Microorganisms | Saccharomyces cerevisiae (yeast) | Acetobacter species (bacteria) |
| End Products | Ethanol and carbon dioxide | Acetic acid (vinegar) |
| Oxygen Requirement | Anaerobic process (no oxygen) | Aerobic process (requires oxygen) |
| Primary Use in Beverage | Production of alcoholic drinks like beer, wine, and spirits | Production of vinegar and flavoring agents |
| Duration | Several hours to a few days | Several days to weeks |
| pH Range | Typically 4.0 to 6.0 | Typically 2.5 to 3.5 |
Introduction to Fermentation Processes
Alcoholic fermentation primarily converts sugars into ethanol and carbon dioxide using yeast, playing a crucial role in producing beverages like beer and wine. Acetic fermentation involves the oxidation of ethanol into acetic acid by acetic acid bacteria, essential for vinegar production. Both fermentation processes utilize distinct microbial pathways that transform organic substrates into valuable biochemicals.
What is Alcoholic Fermentation?
Alcoholic fermentation is a biological process in which yeast converts sugars into ethanol and carbon dioxide under anaerobic conditions, primarily used in brewing and winemaking. This fermentation pathway is essential for producing alcoholic beverages, as it generates alcohol and effervescence while preserving the flavor profile of the ingredients. Understanding the distinction between alcoholic fermentation and acetic fermentation helps you control fermentation outcomes for desired product qualities.
What is Acetic Fermentation?
Acetic fermentation is a biological process where ethanol is oxidized by acetic acid bacteria, producing acetic acid and resulting in the sour taste characteristic of vinegar. Unlike alcoholic fermentation, which converts sugars into ethanol and carbon dioxide using yeast, acetic fermentation involves aerobic conditions and bacteria from the Acetobacter genus. Understanding acetic fermentation helps you control vinegar production and optimize flavor profiles in food and beverage applications.
Key Differences Between Alcoholic and Acetic Fermentation
Alcoholic fermentation primarily converts sugars into ethanol and carbon dioxide using yeast, whereas acetic fermentation transforms ethanol into acetic acid through acetic acid bacteria. The key difference lies in the end products: alcoholic fermentation produces alcohol for beverages like wine and beer, while acetic fermentation generates vinegar with its characteristic acidic taste. Oxygen presence is another differentiator; alcoholic fermentation occurs anaerobically, whereas acetic fermentation requires aerobic conditions.
Microorganisms Involved in Each Fermentation
Alcoholic fermentation primarily involves yeast species, especially Saccharomyces cerevisiae, which convert sugars into ethanol and carbon dioxide. Acetic fermentation is driven by acetic acid bacteria, such as Acetobacter and Gluconobacter species, which oxidize ethanol into acetic acid. Understanding the specific microorganisms in each fermentation process helps optimize your production of alcoholic beverages or vinegar.
Biochemical Pathways: Alcoholic vs Acetic
Alcoholic fermentation converts glucose into ethanol and carbon dioxide through the glycolysis pathway followed by the action of pyruvate decarboxylase and alcohol dehydrogenase enzymes. Acetic fermentation, in contrast, oxidizes ethanol into acetic acid using acetic acid bacteria such as Acetobacter, employing the enzyme alcohol oxidase in an aerobic environment. Your understanding of these distinct biochemical pathways is essential for optimizing fermentation processes in industries like brewing and vinegar production.
Industrial Applications and Products
Alcoholic fermentation is primarily used in the industrial production of beverages such as beer, wine, and spirits, relying on yeast to convert sugars into ethanol and carbon dioxide. Acetic fermentation, on the other hand, is crucial for producing vinegar by oxidizing ethanol into acetic acid using acetic acid bacteria, widely applied in food preservation and flavoring. Your understanding of these processes can guide optimal selection for industries focused on alcohol production or acid-based products.
Environmental Conditions Required
Alcoholic fermentation requires anaerobic conditions with a temperature range of 20-30degC and a pH around 4.0 to 6.0, favoring yeast activity for ethanol production. Acetic fermentation needs aerobic conditions, temperatures between 25-30degC, and a slightly acidic pH of 5.0 to 6.5 to support acetic acid bacteria converting ethanol into acetic acid. Both processes depend on specific oxygen levels and temperature controls to optimize microbial growth and metabolic efficiency.
Impact on Flavor and Quality
Alcoholic fermentation produces ethanol and CO2, creating distinct fruity and sweet flavors crucial for wine, beer, and spirits quality, while acetic fermentation converts ethanol into acetic acid, imparting sharp, vinegar-like sourness that can enhance or spoil the aroma and taste of products like vinegar and kombucha. Alcoholic fermentation enhances complexity and mouthfeel, contributing to a smooth and balanced sensory profile, whereas acetic fermentation impacts acidity and preservation, influencing the tangy and crisp texture of the final product. Controlled fermentation conditions are essential to optimize flavor development and avoid off-flavors, ensuring the quality and consumer appeal of fermented beverages.
Health and Safety Considerations
Alcoholic fermentation primarily produces ethanol and carbon dioxide, requiring controlled conditions to prevent harmful microbial contamination and limit ethanol toxicity, especially in consumable products. Acetic fermentation converts ethanol into acetic acid through Acetobacter bacteria, necessitating careful monitoring to avoid excessive acidity, which can cause irritation or damage if ingested in high concentrations. Proper hygiene, temperature control, and fermentation duration are essential in both processes to ensure product safety and minimize health risks associated with harmful byproducts or contamination.
Alcoholic fermentation vs acetic fermentation Infographic
 libmatt.com
libmatt.com